 |
|
 |
 |
Related Pages |
 |
 |
 |
How to Find a Cancer Treatment Trial 1
This guide will help you to learn about cancer treatment trials that are of potential benefit to you and to decide whether to participate in a particular trial.
|
 |
 |
|
|
 |
Help with Your Clinical Trials Search Results
Unexpected Search Results
The search results page displays a list of trials in PDQ that match the criteria that
you used on the search form. See
About Your Search Results 2
to learn more about the information presented for each trial when you click on the title.
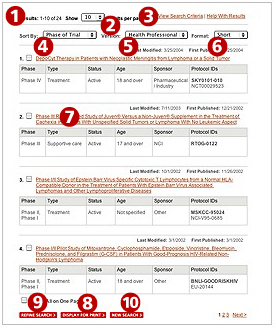
- The top of the page shows the number of trials that matched your criteria.
- Use the "Show" drop down list to change the number of trials displayed on each page.
- Click the "View Search Criteria" link to see the criteria you selected for your search.
- Use the "Sort by" drop down list to reorder the trials according to the field you choose. By default, the trials are ordered according to phase.
For example
phase IV trials will be displayed first followed by
phase III,
phase II, and finally
phase I.
You may choose to have the trials reordered by title, type of trial, status of trial, age range, trial sponsor, and protocol ID.
- Use the "Version" choices to display information designed for either health professionals or patients.
- The "Health Professional" version presents more detailed information and contains technical language.
- The "Patient" version outlines the trial's purpose, treatment plan, and some key eligibility criteria in lay language. It also has links from medical terms to definitions in the
Cancer.gov dictionary 3.
- Both versions provide trial contact information.
- Use the "Format" drop down list to choose how much information to display for each trial.
- The Short format displays the name of each trial, which is a link to the trial's description.
- The Medium format displays all the information in the "Short" format plus additional detailed information about the medical objectives and entry criteria for the trial.
- The Long format displays all the information from the "Medium" format plus details about projected accrual (the expected number of participants), any results that have been published, and contact information.
- The Custom format allows you to select which information from the "Health Professional" version to display. This format is not available for the "Patient " version.
- The title of each trial is a link to the trial's description.
- To print your search results, you must first select one or more trials to display for print. Then click on the "Display for Print" button at the bottom of the page.
You may find that your search results include trials that are not exactly what you want or you may wish to change some of your search criteria. To improve your search, try refining your search criteria or start a new search.
- The "Refine Search" link at the bottom of the search results page takes you back to the advanced form with your original criteria still in place. You may select additional search criteria or change already selected criteria.
- To return to a cleared search form and start a new search, click on the "new search" button at the bottom of the search results page.
|
Glossary Terms
phase I trial
The first step in testing a new treatment in humans. These studies test the best way to give a new treatment (for example, by mouth, intravenous infusion, or injection) and the best dose. The dose is usually increased a little at a time in order to find the highest dose that does not cause harmful side effects. Because little is known about the possible risks and benefits of the treatments being tested, phase I trials usually include only a small number of patients who have not been helped by other treatments.
phase II trial
A study to test whether a new treatment has an anticancer effect (for example, whether it shrinks a tumor or improves blood test results) and whether it works against a certain type of cancer.
phase III trial
A study to compare the results of people taking a new treatment with the results of people taking the standard treatment (for example, which group has better survival rates or fewer side effects). In most cases, studies move into phase III only after a treatment seems to work in phases I and II. Phase III trials may include hundreds of people.
phase IV trial
After a treatment has been approved and is being marketed, it is studied in a phase IV trial to evaluate side effects that were not apparent in the phase III trial. Thousands of people are involved in a phase IV trial.
|
Table of Links
| 1 | http://www.cancer.gov/clinicaltrials/finding/treatment-trial-guide |
| 2 | http://www.cancer.gov/clinicaltrials/advanced-search-form-help/page4 |
| 3 | http://www.cancer.gov/dictionary |
|
 |