 |
 |
Related Pages |
 |
 |
 |
How to Find a Cancer Treatment Trial 1
This guide will help you to learn about cancer treatment trials that are of potential benefit to you and to decide whether to participate in a particular trial.
|
 |
 |
|
|
 |
How to Find Clinical Trials Using the Advanced Search Form
Helpful Hints to Prepare Your Search
Help with Your Clinical Trials Search Results
About Your Search Results
How to Get Help from an Information Specialist
How to Find Clinical Trials Using the Advanced Search Form
The advanced search form allows you to choose from a variety of criteria to develop your search strategy. The following fields on the advanced search form indicate the different criteria you can use to narrow your search:
Use this list to choose the type of cancer being studied in a clinical trial(s). You may choose a type of cancer or you have the option to select "All."
- If you want trials for any type of
solid tumor,
select "Solid tumor, unspecified."
- For trials that treat
metastatic cancer,
select "Metastatic cancer."
After you select a type of cancer, this list will show you the stages/subtypes for the selected cancer. The stage of cancer is the extent of cancer within the body, including whether it has spread. You may select one or more stages/subtypes or "All."
Use this list to choose the type of trial. Some trials fall into more than one category. You may check more than one box or select "All." The following trial types are available:
- Treatment - trials that study potential anticancer treatments, their safety, and their effectiveness.
- Screening - trials that check for disease when there are no symptoms.
- Genetics - trials that study the genetic factors that may influence the development of cancer or the response to cancer treatment.
- Supportive care - trials that study treatments to prevent, control, or relieve complications and side effects and to improve patients' comfort and quality of life.
- Prevention - trials that study ways to prevent disease.
- Diagnostic - trials that evaluate methods of detecting disease.
Use this box to choose whether to search for active trials - those accepting new patients - or closed trials, those no longer accepting new patients.
- The default is for active trials.
- Closed trials might have published information describing what researchers have already studied and learned.
- Closed trials are not searchable by geographic area.
An identification (ID) number that makes it easy to find a specific trial (similar to a book's call number in a library). Use this field only if you know the ID number or partial ID for a specific trial.
- Enter one or more protocol IDs, separating them with commas.
- The search results will list trials that include any of the IDs you entered.
Use this field to search by ZIP Code or by city, state, and country. Enter either your ZIP Code to search for trials in your area or the ZIP Code of another area of interest. By default, searches are limited to within 20 miles of the ZIP Code that you enter. Use the drop down list to change this to choices between 0 and 500 miles.
- The link to "ZIP Code Lookup" pops up a page from the U.S. Postal Service Web site. Find the ZIP Code by entering the address and copy it from the pop up window into the form.
- You can enter a combination of city, state, and country. (States are unavailable for selection if you choose any country other than "All" or "U.S.A.")
- You can select multiple states using the drop down list.
Use this field only if you are looking for trials conducted at a specific hospital or institution.
- Use the "choose from list" link to look up a hospital or institution name.
- When you click on "choose from list," a new window will pop up. You can search for a particular name or look through the list alphabetically. Check the box next to the hospital(s) or institution(s) you want and click the "Select Checked" button at the bottom left. The name(s) will be entered automatically into the form.
- The search results will list trials that include any of the names you selected.
- Use this field to find trials taking place at military or VA hospitals. To find VA facilities, use the "choose from list" and select the letter "V". This will display the VA facilities in the database.
- Select the "NIH Clinical Center (Bethesda, MD)" option to see only the trials located at NIH.
Checking this box limits search results to only those trials added
in the last 30 days.
- New trials are posted to the Web site weekly.
- For example, if the update occurs on June 3, and you select this option any day after that in June, your results will show the trials added on June 3.
Use this list to select one or more types of treatment or interventions used in trials. A list of treatments such as radiation therapy, chemotherapy, surgery, biological therapy, bone marrow transplantation and many more are available for selection. You may select one or more treatments or interventions, or choose "All."
Use the "choose from list" link to look up a drug name.
- When you click on "choose from list," a new window will pop up. You can search for a particular drug or look through the list alphabetically. Check the box next to the drug(s) you want and click the "Select Checked" button at the bottom left. The name(s) will be entered automatically into the form.
- The search results will list trials that include any of the drug names you selected.
- When you check the small box labeled, "Find trials that include all drugs shown," the search results will list trials that include all of the drug names you selected.
- Different drug names - generic, brand/trade, and short names - are equally recognized.
Use this field to select the phase of trial. Most clinical trials are designated as phase I, II, III, or IV, based on the type of questions the trial is trying to answer. You may select one or more trial phases, or choose "All."
- Phase I - trials that test the best way to give a new treatment (for example, by mouth, intravenous infusion, or injection) and the best dose.
- Phase II - trials that test whether a new treatment has an anticancer effect (for example, whether it shrinks a tumor or improves blood test results) and whether it works against certain types of cancer.
- Phase III - trials that compare the results of people taking a new treatment with the results of people taking the standard treatment (for example, which groups have better survival rates or fewer side effects).
- Phase IV - trials that evaluate side effects that were not apparent in the phase III trial.
Use this field if you wish to select a sponsor category. For NCI trials, sponsorship is assigned based on how the trial is reviewed. For all others, assignment is based on who is coordinating or funding the trial. You may select one or more sponsors, or choose "All."
- You can limit your results to trials sponsored by 1) one of the institutes or centers at the
National Institute's of Health 2 (NIH) listed on the drop down list, 2) pharmaceutical companies, or 3) other medical/research institutions.
- Trials sponsored by one of the institutes or centers can be located anywhere (not just the NIH campus).
- Some insurance companies may have restrictions on the trials they will cover. For example, they may cover costs for trials sponsored by some organizations but not others.
Use this list to select a special trial category. You may choose more than one, or select "All." Most patients may prefer to select "All." The following special categories are available:
- NCI-CMS Pilot Project trials - These trials were selected for a
pilot project between the National Cancer Institute (NCI) and the Center for Medicare and Medicaid Services (CMS) 3. Under this pilot project, the CMS will cover the routine and non-routine costs for these trials.
- NCI Avon Award trials - Breast cancer trials funded by the NCI-Avon Progress of Patients Awards program, a partnership between NCI and the Avon Foundation, the charitable arm of Avon Products, Inc.
- CTSU trials - The Cancer Trials Support Unit (CTSU) 4 is a project sponsored by NCI's Cancer Therapy Evaluation Program (CTEP) 5 designed to give physicians and patients greater access to NCI-sponsored, phase III, adult cooperative group clinical trials. Cooperative Group sites located within the United States and Canada are currently eligible for participation in the CTSU. In addition, the CTSU is open to physicians and institutions in the United States who are not affiliated with a Cooperative Group.
- Group C/Treatment IND trials - These trials use Group C-designated drugs, investigational drugs found to have anti-tumor activity which are awaiting final FDA approval and are not yet available on the market.
- Treatment Referral Center (TRC) trials - These trials offer investigational treatments for certain high priority agents or diseases and are available only at NCI-designated cancer centers.
- NIH Clinical Center trials - Trials being conducted at the NIH Clinical Center in Bethesda, MD.
- SPORE trials - Trials funded through NCI's Specialized Programs Of Research Excellence (SPOREs). The goal of the SPORE program 6 is to bring to clinical care settings novel ideas that have the potential to reduce cancer incidence and mortality, improve survival, and improve the quality of life. Laboratory and clinical scientists work collaboratively to plan, design, and implement research programs that impact on cancer prevention, detection, diagnosis, treatment, and control.
- NCI Web site featured trials - NCI-sponsored trials that were highlighted with a special Web article to give them added attention.
Use this field to select medical professionals and researchers who are conducting the trial. Use the "choose from list" link to look up a name.
- If you click on "choose from list," a new window will pop up. You can search for a particular name or look through the list alphabetically. Check the box next to the investigator(s) you want and click the "Select Checked" button at the bottom left. The name(s) will be entered automatically into the form.
- The search results will list trials that include any of the names you selected.
Use this field if you wish to select an academic hospital, research institute, pharmaceutical company, cancer center, or cooperative group responsible for coordinating the trial. Use the "choose from list" link to look up the name. This field may not be applicable for most users.
- Cooperative groups are NCI-funded groups of institutions, researchers, and community physicians. More information is available in
NCI's Clinical Trials Cooperative Group Program 7.
- If you click on "choose from list," a new window will pop up. You can search for a particular name or acronym or look through the list alphabetically. Check the box next to the organization(s) or group(s) you want and click the "Select Checked" button at the bottom left. The name(s) will be entered automatically into the form.
- The search results will list trials that include any of the names you selected.
Helpful Hints to Prepare Your Search
It is helpful if you first talk to your health care provider and gather as much information as possible about your particular situation, such as the specific type and stage of cancer, the type of trial that might be relevant (treatment, diagnostic). The following information will help you prepare your search and evaluate the results.
- You may wish to start with the
basic 8 (short) form and then refine your search by using the
advanced 9 (long) form.
- In the basic form, you must choose a "Type of Cancer." "Stage/subtype", "Type of Trial" and "Location of Trial" are optional.
- In the advanced form you can create your search using more detailed information. You can select multiple criteria to narrow your search or you can look up a specific trial. All fields are optional.
- The fields on the search form specify the different criteria you can use to narrow your search.
- The more criteria you choose, the fewer results you are likely to get.
- You may skip any criteria that are unknown or not applicable. The default value for every field is "All."
Help with Your Clinical Trials Search Results
The search results page displays a list of trials in PDQ that match the criteria that
you used on the search form. See
About Your Search Results 10
to learn more about the information presented for each trial when you click on the title.
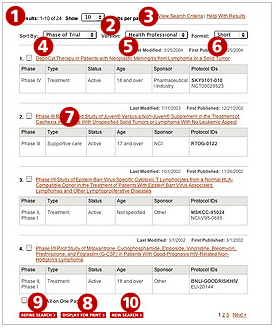
- The top of the page shows the number of trials that matched your criteria.
- Use the "Show" drop down list to change the number of trials displayed on each page.
- Click the "View Search Criteria" link to see the criteria you selected for your search.
- Use the "Sort by" drop down list to reorder the trials according to the field you choose. By default, the trials are ordered according to phase.
For example
phase IV trials will be displayed first followed by
phase III,
phase II, and finally
phase I.
You may choose to have the trials reordered by title, type of trial, status of trial, age range, trial sponsor, and protocol ID.
- Use the "Version" choices to display information designed for either health professionals or patients.
- The "Health Professional" version presents more detailed information and contains technical language.
- The "Patient" version outlines the trial's purpose, treatment plan, and some key eligibility criteria in lay language. It also has links from medical terms to definitions in the
Cancer.gov dictionary 11.
- Both versions provide trial contact information.
- Use the "Format" drop down list to choose how much information to display for each trial.
- The Short format displays the name of each trial, which is a link to the trial's description.
- The Medium format displays all the information in the "Short" format plus additional detailed information about the medical objectives and entry criteria for the trial.
- The Long format displays all the information from the "Medium" format plus details about projected accrual (the expected number of participants), any results that have been published, and contact information.
- The Custom format allows you to select which information from the "Health Professional" version to display. This format is not available for the "Patient " version.
- The title of each trial is a link to the trial's description.
- To print your search results, you must first select one or more trials to display for print. Then click on the "Display for Print" button at the bottom of the page.
You may find that your search results include trials that are not exactly what you want or you may wish to change some of your search criteria. To improve your search, try refining your search criteria or start a new search.
- The "Refine Search" link at the bottom of the search results page takes you back to the advanced form with your original criteria still in place. You may select additional search criteria or change already selected criteria.
- To return to a cleared search form and start a new search, click on the "new search" button at the bottom of the search results page.
About Your Search Results
There are two versions of each trial description - one designed for patients and another for health professionals. The patient version is written using non-technical language and the health professional version is written using technical terminology. You may move between the two by using the tabs near the top of the page.
When using the advanced search form, your trial results will be displayed using the health professional version of the trial description along with a more technical title. You will also see an alternate title, which is the patient-friendly or non-technical title.
The basic trial information provides detailed information about the goals of the trial and summarizes who is eligible and how the trial will be conducted. You or your health care provider can use the contact information to find out more about the trial. Each trial description contains the following sections:
-
A detailed description of the trial's goals.
A list of the requirements a patient must meet to participate in the trial. There are three categories:
- Disease Characteristics - type and stage of cancer.
- Patient Characteristics - age, medical conditions, etc.
- Prior/Concurrent Therapy - for example, whether the patient has already had chemotherapy.
The number of patients expected to participate in the trial.
A summary of the treatment plan.
Reference citations to publications resulting from the trial, if available.
A list of one or more academic hospitals, research institutes, pharmaceutical companies, cancer centers, or cooperative groups responsible for coordinating the trial.
The people or organizations conducting the trial. They can provide more information about the trial, including eligibility, the enrollment process, and other details.
Note: Some of the trials in PDQ are cancer trials from the
National Library of Medicine's (NLM) ClinicalTrials.gov database 12. Because these trials are obtained from another database, they may not contain all of the categories of information listed above. Also, the trials from the
ClinicalTrials.gov 13 database contain the same text in both the patient and health professional versions.
 |
How to Get Help from an Information Specialist
If you do not want to search for trials on your own, you may get help from an experienced information specialist or use the live, online help available through the National Cancer Institute's (NCI) Cancer Information Service (CIS).
- To get help from a specialist, call NCI's Cancer Information Service (CIS) at 1-800-4-CANCER (1-800-422-6237) or TTY: 1-800-332-8615 between 9:00 a.m. and 4:30 p.m. local time, Monday - Friday. The CIS specialist will ask about your specific situation and provide information relevant to your needs
- Live, online help is available through the CIS
LiveHelp 14 instant messaging service Monday - Friday, 9:00 a.m. to 11:00 p.m. U.S. Eastern time. Find the CIS "Need Help" link in the sidebar at
the left of most pages of NCI's Web site.
|