
Why the National Biospecimen Network?
| This module presents the rationale for the National Biospecimen Network (NBN) system,
describes how it is expected to differ from extant tissue resources, and identifies the resource
requirements and challenges associated with the development of an NBN that will be of
maximum utility to researchers. This module focuses primarily on researcher needs, and seeks to
provide detailed requirements for key components of the NBN. Recommendations for
implementation are discussed in subsequent modules. |
1.1 Background
Participants in the groundbreaking National Dialogue on Cancer (NDC) Research Team Forum I
held in March 2002 identified access to appropriately collected and annotated tissue as a critical
need for fully capitalizing on new genomic and proteomic technologies to accelerate progress
against cancer.1 The group identified the lack of such access as one of the major barriers to
realizing the promise of developing targeted cancer diagnostics, preventives, and therapies. The
opinions expressed at the Forum I meeting echoed previous public comments of prominent
researchers, and the conclusions of several advisory committee reports to the NCI in a variety of
cancer research areas, including brain tumor, leukemia, lymphoma, myeloma, lung cancer, and
gynecologic cancers.2
Several recent examples illustrate how access to relatively large numbers of tissue samples has
played a pivotal role in oncology drug development, and these examples underscore the likely
benefits from implementing a standardized system by which researchers and clinicians may gain
access to biospecimens and associated data. The development of trastuzumab (Herceptin®) is a
success story that demonstrates the potential of biomarkers in the rational design and
development of cancer drugs that could not have been realized without access to tissue samples.
The clinical benefits of trastuzumab would almost certainly have been insufficient for FDA
approval if the agent had been tested in unselected patient populations (Appendix A). The
Gleevec® story demonstrates how alternative uses for a drug can be discovered through
investigations conducted with tissue samples. Gleevec® originally was developed for the
treatment of chronic myeloid leukemia. However, screening of tissue samples for c-kit activation
identified gastrointestinal stromal tumor patients as potential clinical benefactors (see Appendix B). Finally, as researchers unravel links between molecular pathways and specific cancers and
treatments, thereby discovering yet untold uses for many existing therapies, standardization of
tissue collection and analysis will become increasingly important for linking various independent
observations. Tissue samples played a pivotal role in the development of laser capture microdissection, a breakthrough technique that facilitates the precise, reproducible, and accurate
transfer of tissues for analysis (see Appendix C).
In response to the challenge articulated by the NDC Research Team—a self-selected group of
individuals involved in cancer research, drug development, delivery, and commercialization, as
well as representatives from patient advocacy organizations—met in Washington, D.C. on
August 26-27, 2002, on January 7, 2003, and again as part of the NDC Forum II meeting on
March 5-7, 2003, to further investigate the barriers involved in tissue access and to explore
possible avenues for improvement. This group, the NDC Tissue Access Working Group
(TAWG), sought to design an approach that could meet the Nation’s research needs for
biological specimens, and to present options for moving forward. It was at the Forum II meeting
that Cathy Ratcliffe from the National Translational Cancer Research Network (NTRAC)
presented the United Kingdom (UK) experience with the development of their National Cancer
Tissue Resource (NCTR), and TAWG members were provided a copy of the NCTR strategic
plan.3 The UK experience effectively accelerated the United States. Blueprint development
process by several months.
During their deliberations, the NDC TAWG members reinforced the conclusion that the
development of a national tissue resource, although ambitious, is necessary to realize the promise
of genomics and proteomics for the prevention and cure of cancer and other diseases.
Unparalleled advances in dissecting the genetic changes and molecular mechanisms that
ultimately produce cancer have provided, for the first time, compelling reasons to pursue targetspecific
interventions. It is now well-recognized that there is a high degree of disease
heterogeneity, that sample characteristics and preparation will impact results, and that large
samples are required for robust design and rigorous conclusions. To reflect this understanding,
the NDC TAWG defined its goal as:
"to establish a national, pre-competitive, regulatory compliant and geneticprivacy
protected, standardized, inclusive, highest quality network of biological
sample(s) banks; supported by and developed via novel financial and other
partnerships with cancer survivors and advocates, the private sector and nonprofit
organizations as appropriate; that is shared, readily accessible, and
searchable using state-of-the-art informatics systems (e.g., amenable to
molecular profiling capability)."4
Building on the ideas and vision shaped by the NDC TAWG, an effort was initiated to create a
Design and Engineering Blueprint for an NBN (the "NBN Blueprint"), a biospecimen resource
envisioned for optimizing and accelerating the development of new interventions for cancer. The
major goals of the NBN Design Team were to articulate the unmet needs that the NBN seeks to
address, to clarify the NBN customer base, including the role of patients and advocates as well as
commercial interests, and to describe the desired processes that the NBN would engage in to
meet its goals. The Design Team met in Bethesda, MD on May 28, 2003, to achieve a collective understanding of the purpose of the NBN Blueprint document and the process for its development, to agree on the objectives, key questions, issues, concerns, and types of recommendations to be addressed in each module, and to agree on a general framework for
completing the NBN Blueprint. The May 28 meeting also gave participants an opportunity to
discuss the challenges (including institutional and other barriers) as well as opportunities to
integrate with and adopt best practices from existing systems in the United States and from the
UK’s NCTR model.
The NBN Design Team’s deliberations were enhanced by site visits in May 2003 to tissue
resource operations considered to provide state-of-the-art facilities with optimal data access
processes as well as an intense period of conference calls during the months of June-August
involving the Design Team members and advisors for each particular module topic. The NBN
Design Team also benefited from the RAND evaluation of selected existing tissue resources: an
exercise that included description of the types of tissue users and distribution practices, and
queries to ongoing tissue resource managers of any unmet needs or quality control (QC) issues
(see Appendix D for interview instrument). A questionnaire was administered at the American
Association for Cancer Research meeting in Washington, D.C. in July 2003 that collected
information from meeting participants about their anticipated uses for and reactions to the
development of the NBN, as well as their willingness to pay (see Appendix E). This information
also assisted the Design Team in their deliberations.
On July 28-29, 2003, the NBN Design Team and invited experts convened to discuss
overarching issues, integration of the modules, and final recommendations that should appear in
the Blueprint. (See Appendix F for the list of participants to the July 28-29 NBN Blueprint
meeting.) Earlier versions of this Blueprint have undergone extensive review by outside experts
selected by Constella Health Sciences in consultation with the sponsors, to help ensure the
accuracy and relevancy of information provided in this report and to capture the broadest
representation possible from diverse viewpoints.
The Design Team identified five primary areas where the NBN could bring value to researchers:
(1) Standardized collection of large numbers of fresh/frozen cancer specimens; (2) Accurate,
highly standardized clinical annotation and associated data; (3) Prompt and equitable specimen
accessibility; (4) Informatics platforms to facilitate sharing of data and results; and (5) Protection
of patient privacy. Items one and two specifically address the variation in collection procedures
and annotation that currently inhibits uniform comparisons among tissue collections, and items
three and four address the frustration expressed by many researchers and advocacy groups about
the lack of biospecimen resource sharing.5
Additionally, the Design Team envisioned the NBN as serving a number of well-defined niches
for researchers. Full implementation of the NBN might well see centralized, advanced analyses
of a subset of specimens, as well as the collection of standardized longitudinal data for a high
percentage of specimens. Not all researchers will have the same needs; thus, some subsets of
samples will be accompanied by more extensive longitudinal data, while other subsets will have undergone one or more types of advanced analyses; combinations of these subsets also will exist.
However, all specimens should be characterized by the major areas of value outlined above.
 Top of Page Top of Page
1.2 Purpose of the National Biospecimen Network
The NBN is envisioned to be the first national, standardized tissue resource in the United States
designed to facilitate genomic and proteomic research, with open access to cancer researchers
across the country. Although several countries—including the UK6, Iceland,7 and Japan 8—are
investing in nationally coordinated specimen collection, banking, and dissemination systems
specifically designed to support genomic and proteomic research, no effort has been attempted
on a comparable scale in the United States.
The NBN can facilitate a range of scientific activities that could lead to new genomic- and
proteomic-based interventions for cancer, including target identification and validation, the
development of new biomarkers and diagnostics, and pharmacogenomic analyses. Recent
breakthroughs in the biomedical sciences have produced a wealth of new knowledge about the
diagnosis, treatment, and prevention of cancer. Biospecimens, which historically have been a key
element of cancer research,9 have now assumed a central position in the application of genomic
technologies to new interventions for cancer. As researchers unravel the roles of particular biomarkers and cellular pathways in specific cancers, biospecimens will help link observations
from the laboratory to disease processes observed in the physiological setting.
As shown in Figure 1-1, the NBN is designed to provide a link between epidemiological
investigations that identify genetic and environmental risk factors and clinical trials that directly
test new interventions for cancer. Several existing efforts fall within the scope of the sectors
shown in Figure 1-1. For example, the UK Biobank, Biobank Japan, and DeCode Genetics in
Iceland support large-scale genetic epidemiology efforts, whereas the U.S. Cooperative
Oncology Groups collect many biospecimens in the context of clinical trials.10 A recent initiative
to develop an NCTR in the UK shares several key elements with the NBN concept. As discussed
further in an accompanying report on "best practices,"11 several existing repositories include
individual aspects of the NBN vision; however, the NBN would uniquely integrate a specific
combination of features needed to translate basic genomic and proteomic research into clinical
discoveries for cancer patients in the United States.
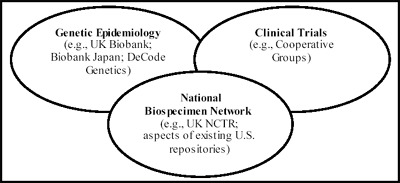
Figure 1-1: The Relationship of the NBN to Other Biospecimen Collection Initiatives
While the postgenomic era holds great promise for the use of biospecimens, it also is changing
the biospecimen needs of cancer researchers. It is estimated that more than 300 million
specimens representing more than 150 million cases currently are stored in the United States,
with over 20 million new specimens added each year.12 However, many of these samples are not
collected, stored, or annotated in a manner that is compatible with genomic analysis.
Furthermore, recent Federal regulations, such as the Health Insurance Portability and Accountability Act of 1996, have reconfigured privacy issues. Researchers often find themselves
navigating a complex maze of intellectual property rights, liabilities, and other sociological
barriers that currently impede the sharing of tissue samples for research and discourage clinical
investigations. As a consequence, current programs and practices collectively fall short of
meeting the research community’s most pressing needs in genomic and proteomic research.
1.2.1 Researcher Needs
Cancer researchers have called repeatedly for biospecimens to be collected using standardized
protocols, so that results can be reproducible and comparable. They seek greater research data
accessibility through an open, Web-based platform, while remaining committed to the
proposition that the collection and use of biospecimens and associated data must meet the highest
possible ethical standards for protecting the privacy and confidentiality of the donor. They also
recognize that the usefulness of biospecimens is maximized if accompanied by relevant
demographic, social history, clinical, pathology, and longitudinal data, as well as genomic and/or
proteomic data. A searchable, Web-based bioinformatics system therefore is seen as crucial for
facilitating scientific discovery. Many investigators also have expressed a desire for the services
that accompany tissue sample analysis, such as tissue microarrays and DNA or RNA assays. The
suggested approach for incorporating all of these features into an NBN is described in this
Blueprint report.
The potential sources for biospecimens are expected to be derived primarily from academic
medical centers and community hospitals. The potential users would be primarily scientists and
researchers at academic institutions, government agencies, and biotech and pharmaceutical
companies. The potential uses of biospecimens and associated data are many, including for the
following purposes:
- Target- and validation-discovery of molecular correlates
- Primarily using RNA or protein analysis methods (large and small scale)
- Genomic analysis
- Mutation screening
- Loss of heterozygosity and amplification studies
- Methylation studies
- Validation of diagnostic or therapeutic antibodies, or nucleic acid probes
- Pharmacogenomic analysis
These areas involve both major and minor cancer types, as well as specimens from primary and
metastatic cancer sites. Descriptions of how particular NBN constituencies might use
biospecimens are found in Appendix G. When determining which products to provide to its
users, NBN must address the tissue amounts required, the tissue quality required (e.g., ranging
from standard clinical quality to RNA grade to protein grade), the types and numbers of tissues
required (e.g., primary and metastatic sites), and the format of tissue (e.g., sections of tumors to
tissue microarrays) that would be most useful to researchers for each of the above purposes.
1.2.1.1 Commercial Interests
Pharmaceutical companies comprise an important component of the customer base for the NBN.
Easier access to well-annotated cancer samples could help make oncology more attractive to
pharmaceutical companies and will enhance investment in developing anticancer therapies.
Advances in genomics are likely to continue to segment histologically defined cancers into
better-defined subsets, which may result in smaller market segments. However, these smaller but
genetically defined market segments may offer the possibility for better patient responses, and
ultimately may pose less risk to private sector investments if identified during the early stages of
the drug development process. Other commercial users would include a broad range of
companies developing predictive and diagnostic products directed at cancer and other diseases.
These include companies developing diagnostic/prognostic/therapeutic antibodies, as well as
companies testing new technologies. Commercial companies need access to well-defined clinical
samples in order to fully develop targeted agents and new technologies. The NBN will provide
access to biospecimens for these industry customers.
1.2.1.2 Academic Centers
Academic medical centers will be a principal source of the operational and technique-related
expertise for the NBN, as the vast majority of current research resources are located at academic
health centers. Academic researchers, by virtue of their numbers, will also constitute the primary
user base of the NBN.13 Currently, many tissue access systems provide access to specimens and
data only for researchers within the institution in which specimens are collected.14 The NBN
seeks to broaden access and standardize procedures for obtaining specimens.
While a number of biospecimen resources exist at selected government, academic, nonprofit and
for-profit institutions in the United States, there is no national, standardized, openly accessible
biospecimens repository and database that is available to researchers who are interested in
pursuing genomic or proteomic research. The NBN Blueprint will allow for the development of a
system that will increase access across the country to these important biospecimens and
associated data, while at the same time streamlining the collection and analysis of these samples
from existing resources. In fact, elements of current biospecimen resources will be incorporated
into the NBN through a "best practices" framework. The resource is intended to be shared openly
among researchers at public and private institutions throughout the country, without the
competitive or intellectual property constraints that are often barriers to resource sharing.
Although a large number of biospecimens exist in repositories today (there currently are
approximately 350 organizations), materials are in various states of usefulness and readiness. In
addition, no overarching standards exist, fresh/frozen tissue is not always readily available, and variations in specimen collection procedures and annotation across specimen collections are the norm (see Table 1-1).15
Preliminary findings from the RAND study16 suggest that, while all studied repositories collect
paraffin-embedded samples, some repositories have a relatively small collection of fresh frozen
samples, and the clinical and longitudinal annotation is uneven. Although samples are used for
genomics and proteomics studies after distribution, most repositories do not proffer
genomics/proteomics data. The variability (or lack) of appropriate donor-informed consent and
sample tracking capabilities limits resource utility. Finally, repository design is integrally linked
to its original collection objectives, resulting in little cross-repository standardization.
Table 1-1: Summary of Current Limitations and the Ideal NBN Prototype
| Limitations of Existing Systems |
Ideal NBN Prototype |
| Wide variation in tissue collection, processing, and storage techniques, and difficulty obtaining sufficient samples for large-scale genomic and proteomic studies of rare cancers |
Single, nationally coordinated network of pathological and normal tissue collection, employing standardized procedures for storage and distribution, as well as collection of associated clinical data |
| Nonuniform (or nonexistent) bioinformatics systems that are incapable of remote searching and data entry |
Coordinated and centralized bioinformatics system for all aspects of specimen and data collection and dissemination |
| Restricted access to researchers outside institution at which specimens are collected |
Extensive, external specimen-sharing is required of NBN collection centers on a national scale |
| Reluctance to share exhaustible specimen supply |
Emphasis on collecting inexhaustible data from specimens |
| Consent procedures that are variable and may be insufficient for future genomics/proteomics research |
Standardized consent for all specimens tailored to genomic and proteomic studies |
As summarized in Table 1-1, the ideal NBN prototype is to have (1) a single, nationally
coordinated network of pathological and normal tissue collection, employing standardized
procedures for storage and distribution and collecting associated pathological, clinical,
demographic, social history, and longitudinal data and (2) a coordinated and centralized bioinformatics system for all aspects of specimen and data collection and dissemination. The
NBN is envisioned to have extensive sharing of specimens by NBN collection centers on a
national scale, an emphasis on collecting inexhaustible data from specimens, and more or less
standardized consent of all specimens tailored to genomic and proteomic studies. Like the UK
NCTR, management of the databases and access to them would be monitored by an oversight
body, whose function will be to safeguard the interests of all participants. The resource will be
available to scientists and medical researchers, but there will be strict controls in place to protect
the confidentiality of participants.
The NBN does not anticipate supplanting the existing tissue collection resources in the United
States; rather, it seeks to fill a niche not served by current resources. Existing systems vary by
the nature and intent of their collection protocols, the extent of their data, and their consent
procedures. Some existing systems will align well with the goals of the NBN. Such systems may
have an interest in participating in the NBN, and their involvement and expertise will be
welcomed. Specialized Programs of Research Excellence (SPORE) grantees and Cooperative
Group programs, for example, offer many of the characteristics that the NBN desires, and may
have unmet needs that may be filled by alliances with the NBN. (See 3. Biospecimen and Data
Collection and Distribution and 6. Governance and Business Models for additional information
on this issue.)
Meaningful, broad molecular profiles of cancers can be developed optimally from tissues and
clinical data that are collected using rigorous, highly standardized procedures. Some existing
systems may not be candidates for genomics/proteomics research or future advanced technology
purposes, but they are valuable in their own right. For example, banks like the National Surgical
Adjuvant Breast and Bowel Project clinical trial bank are invaluable for their tight linkage
between detailed clinical information and tissues; however, most of the samples were collected
some time ago and are mostly fixed tissues embedded in paraffin. Similarly, the Armed Forces
Institute of Pathology offers great depth and expertise in pathology diagnosis from fixed tissues,
but it is not a state-of-the-art frozen tissue bank with validated clinical annotation, and never was
intended to be such. These existing systems will continue to serve their specific users’ needs.
 Top of Page Top of Page
1.3 NBN Requirements
Access to appropriately collected and annotated biospecimens is critical to accelerating progress
against cancer in the postgenomics/proteomics era. Paraffin-embedded tissue is adequate for the
clinical diagnosis of a specific cancer in an individual patient, and someday, with the advent of
new technologies, it also may be used for a wide range of genetic studies. However, many stateof-
the-art, molecular genetics-based technologies initially require fresh/frozen tissues, and
successful identification and credentialing of drug targets or confirmation of diagnostic markers
often depend on connecting tissue samples with a patient’s characteristics at initial presentation
and after appropriate followup. Additionally, what is developed as the optimum system today
may not be what is needed after 5 years. Therefore, any system must be forward thinking,
capable of expansion, and flexible, and it must anticipate potential technologies that are yet to be
developed. The NBN system must have the capacity to grow as the information base grows and
evolve as technologies advance.
It is the goal of the NBN to provide researchers from industry, government, and academe with a
standardized, inclusive, high-quality network of biological samples that is shared, readily
accessible, and searchable, using state-of-the-art informatics systems. The NBN must understand
current and anticipated needs of all involved research communities, and it must provide a
product that researchers will both desire and use. Output must be broadly available and readily
accessible to users. Longitudinal clinical data must be periodically updated and should include
data points focusing on therapeutic modalities, response measures, and outcomes. The system
must incorporate high levels of QC, as the quality of the biospecimens and the accuracy of the
resulting data will determine how relevant and usable the samples and data are to researchers.
To meet researchers’ needs, the NBN must collect from patients with cancer sufficient numbers
of tissues, blood, serum, and plasma in a manner that maintains the architecture of the tissues and
the molecular integrity of DNA, RNA, and proteins in the biospecimens. The NBN should
collect and provide detailed clinical (including longitudinal) and eventually genomic data for
biospecimens, in addition to providing access to the biospecimens themselves. Efficient
distribution of biospecimens and data to researchers would require an equitable peer review
system for biospecimens and an integrated, searchable bioinformatics system for data. Long-term
preservation of data would also need to be addressed.17
The Design Team identified the following, overarching requirements in order for the NBN to
meet researcher needs:
Biospecimen and Data Collection and Distribution
- Biospecimens for banking (collected for storage in and distribution by the NBN) should
be obtained only after all patient diagnostic needs have been met, and should be subject
to appropriate bioethical structures and procedures, to ensure patient protection.
- The repository should consist of high-quality biospecimens appropriate for genomic and
proteomic studies, and the type of biospecimens stored in the repository should be
determined by an ongoing review of researcher needs.
- Annotation data (clinical, pathological, demographic, and social history) should be
accurate, quality-controlled, and standardized across collection sites.
- The collection of longitudinal data should be strongly supported, and should include
relevant biomarker measurements, if available. It is recognized that the costs for these
data are likely to be high, and success will require innovative solutions.
- Genomic and/or proteomic testing may be performed on a subset of biospecimens by the
NBN. Both the testing and specific subsets should be responsive to the needs of the users
and flexible to changes in the research environment.
- The NBN should have a comprehensive representation of a broad diversity of disease and
human populations. Biospecimen donors therefore should include a broad range of
ethnicities, socioeconomic groups, and other demographic subgroups.
Bioinformatics and Data Management
- The repository should support open research access and be searchable and mineable via the
Internet and incorporate computational analysis tools. The technology should be amenable
to sharing appropriate clinical and longitudinal data, and at the same time should protect
the donors’ privacy and confidentiality. The repository should be available to a broad
researcher base, and should associate clinical and experimental data with the specimens.
- The database should support integration and expansion, by establishing strict standards
for data contributors and developing platforms that will expand and extend as the science
grows. It should address the different vocabularies and data collection structures inherent
among scientific communities (e.g., genomics, pathology) using common data elements.
- The database should support the exchange of information; it should capture data
generated through use of the resource (both primary data and data interpretations), but
should share and restrict data according to specific rules established by the NBN.
- Although likely to be challenging, it is important that validated, investigator-derived data
be returned to the NBN and linked back to original NBN tissue samples. An expanded
dataset, created by the return of this experimental data to the NBN, could then be made
available to all investigators.
- The architecture should have the ability to "scale" as the volume of data increases, and it
should have the ability to "extend" as datasets and types of data change. The architecture
should provide interfaces that enable the construction of data mining and extraction tools,
providing a comprehensive computational and data analysis environment.
Communications
- The conduct of a broader survey of the potential user population should be a priority, to
develop a more detailed and accurate picture of potential research usage trends, the need for
additional services, cost sensitivities for tissues and data, and advanced analysis services.
- Data and nomenclature standards being created for many types of research results should
be incorporated into the NBN protocols.
Governance and Business Models
- The system must be highly flexible and capable of expanding as the science progresses.
- Development and standardization of collection, processing, storage, and distribution
procedures, as well as QC and quality assurance monitoring, should be of paramount
importance at all stages of the process, to allow for comparison of specimens from
various collection sites.
- Data and samples should be distributed in a clearly articulated and equitable fashion,
based primarily on the quality of the proposed research. Access to biospecimens should
be controlled by a neutral, streamlined peer review system that is facilitated by a
Biospecimen Utilization Review Committee. The tissue access system must include
timely review of requests and distribution of samples, with minimal administrative
burden.
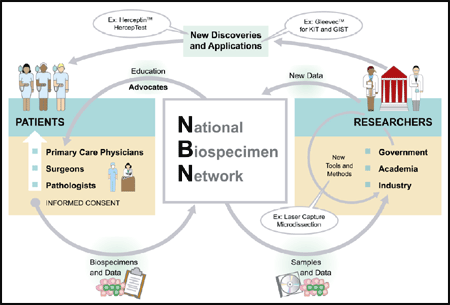
Figure 1-2. An Overview of the National Biospecimen Network
In short, the NBN seeks to create a data and tissue repository that provides aggregated, mineable
information from a large number of biospecimens collected on a national basis. NBN specimens
and data are expected to be highly valued and accessible to cancer researchers in both the private
and public sectors. As depicted in Figure 1-2, researchers need tissue specimens and associated
data as the basis for scientific discovery. The process will begin with the patient, who is the
potential donor of this precious material. Voluntary health organizations can help by educating
the population about the benefits of tissue donation, so that the concept is not a foreign one at an
inopportune time (i.e., when a patient is diagnosed with a possibly fatal disease). After the
patient provides informed consent, pathologists and surgeons will be involved in collecting,
processing, and storing the specimen and providing associated data. The biospecimens and data
will enter the NBN network; the NBN then plays a role in distributing the samples and associated
data to researchers for use. Researchers will be invited and encouraged to return data derived
from the NBN sample back to the NBN, in order to build up the national resource. The
requirements proposed in this module should be considered long-term goals, with certain
components of the NBN expected to become available over time.
|




