 |

Trastuzumab/Chemo Improves Disease-Free Survival in Early-Stage Breast Cancer
The combination of the targeted agent trastuzumab (Herceptin) and standard chemotherapy cuts the risk of HER-2-positive breast cancer recurrence by more than half compared with chemotherapy alone, the National Cancer Institute (NCI) announced yesterday. The result comes from two large, NCI-sponsored, randomized trials testing, as adjuvant therapy, a trastuzumab/chemotherapy combination against chemotherapy alone in women with invasive, early stage, HER-2 positive breast cancer.
The Data Monitoring Committees (DMC) overseeing the trials' combined analysis recommended that the results of a recent, combined interim analysis be made public because the studies had met their primary endpoint of increasing disease-free survival in patients receiving combination therapy. There was also a statistically significant improvement in overall survival with the trastuzumab/chemotherapy combination.
Read more 


Imaging: An Integral Tool on the Path to 2015
One of the biggest changes in biomedical research over the past decade is how we view the group of diseases collectively known as cancer. We are moving beyond the notion of cancer as a disease that affects a single tissue or organ; rather, we are increasingly viewing it as a disruption of molecular mechanisms. This is allowing us to make important strides toward more individualized and targeted interventions, based on factors such as genetic polymorphisms, aberrant signal transduction pathways, or how patients respond in real time to a particular therapy. Although there are a number of new tools that are aiding these shifts, imaging technologies in particular are playing a central role.
In fact, whether it's as a minimally invasive screening tool, a surrogate marker for clinical endpoints in clinical trials, or a method of guiding the delivery of treatment, imaging will be an indispensable tool in the march toward the 2015 goal of eliminating the suffering and death due to cancer.
NCI had recognized that biomedical imaging was a critical area for future development and emphasis, establishing in 1997 the Biomedical Imaging Program - now called the Cancer Imaging Program (CIP). More recently, we established the Molecular Imaging Program (MIP) within the NCI Center for Cancer Research (CCR). Both programs, along with initiatives such as NCI's collaboration with the American College of Radiology Imaging Network to conduct imaging-focused clinical trials, are just some of the creative ways the institute - guided by the invaluable advice of the research and clinical communities - is bolstering the dramatic advances being made in the imaging sciences.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |

Trastuzumab/Chemo Improves Disease-Free Survival in Early-Stage Breast Cancer
The combination of the targeted agent trastuzumab (Herceptin) and standard chemotherapy cuts the risk of HER-2-positive breast cancer recurrence by more than half compared with chemotherapy alone, the National Cancer Institute (NCI) announced yesterday. The result comes from two large, NCI-sponsored, randomized trials testing, as adjuvant therapy, a trastuzumab/chemotherapy combination against chemotherapy alone in women with invasive, early stage, HER-2 positive breast cancer.
 The Data Monitoring Committees (DMC) overseeing the trials' combined analysis recommended that the results of a recent, combined interim analysis be made public because the studies had met their primary endpoint of increasing disease-free survival in patients receiving combination therapy. There was also a statistically significant improvement in overall survival with the trastuzumab/chemotherapy combination.
The Data Monitoring Committees (DMC) overseeing the trials' combined analysis recommended that the results of a recent, combined interim analysis be made public because the studies had met their primary endpoint of increasing disease-free survival in patients receiving combination therapy. There was also a statistically significant improvement in overall survival with the trastuzumab/chemotherapy combination.
"For women with this type of aggressive breast cancer, the addition of trastuzumab to chemotherapy appears to virtually reverse prognosis from unfavorable to good," says Dr. Edward Romond, study chair for one of the two trials led by the National Surgical Adjuvant Breast and Bowel Project (NSABP).
"These findings confirm that we now have a very potent weapon against the recurrence of cancer cells that overexpress HER-2," adds Dr. Edith A. Perez, who chaired the other trial, led by the North Central Cancer Treatment Group (NCCTG).
Trastuzumab, manufactured by Genentech, Inc., specifically targets the HER-2 protein, which is overexpressed in approximately 20 to 30 percent of breast cancers. HER-2-positive tumors are not only more aggressive than tumors that do not overproduce HER-2, they also are more likely to recur. Trastuzumab is approved by the FDA for use in women with HER-2 positive metastatic breast cancer. According to Dr. JoAnne Zujewski, of NCI's Cancer Therapy Evaluation Program, these are the first trials to show a benefit for trastuzumab as breast cancer adjuvant therapy.
The DMC's interim analysis included data on 3,300 of the 5,000 patients enrolled in the trials. Participants - all of whom had small tumors removed surgically with or without radiation - were enrolled in the trials between February 2000 and April 2005. Patients were randomized to receive a regimen of doxorubicin and cyclophosphamide followed by paclitaxel, or doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab. Most patients' cancer had spread to the lymph nodes.
"Even those who anticipated a positive result from these trials will be surprised at the magnitude of the finding - a 52-percent decrease in disease recurrence," says Dr. Zujewski. "That's a significant difference that is rarely seen in a clinical trial."
Additional analyses will allow the trial leaders to perform a more thorough risk/benefit analysis. In the interim analysis, the likelihood of congestive heart failure (CHF) in women receiving the trastuzumab/chemotherapy combination was increased by 3 to 4 percent, compared with a less than 1 percent CHF rate in those treated with chemotherapy alone.
By Carmen Phillips
|
 |
 |

Imaging: An Integral Tool on the Path to 2015
One of the biggest changes in biomedical research over the past decade is how we view the group of diseases collectively known as cancer. We are moving beyond the notion of cancer as a disease that affects a single tissue or organ; rather, we are increasingly viewing it as a disruption of molecular mechanisms. This is allowing us to make important strides toward more individualized and targeted interventions, based on factors such as genetic polymorphisms, aberrant signal transduction pathways, or how patients respond in real time to a particular therapy. Although there are a number of new tools that are aiding these shifts, imaging technologies in particular are playing a central role.
In fact, whether it's as a minimally invasive screening tool, a surrogate marker for clinical endpoints in clinical trials, or a method of guiding the delivery of treatment, imaging will be an indispensable tool in the march toward the 2015 goal of eliminating the suffering and death due to cancer.
NCI had recognized that biomedical imaging was a critical area for future development and emphasis, establishing in 1997 the Biomedical Imaging Program - now called the Cancer Imaging Program (CIP). More recently, we established the Molecular Imaging Program (MIP) within the NCI Center for Cancer Research (CCR). Both programs, along with initiatives such as NCI's collaboration with the American College of Radiology Imaging Network to conduct imaging-focused clinical trials, are just some of the creative ways the institute - guided by the invaluable advice of the research and clinical communities - is bolstering the dramatic advances being made in the imaging sciences.
For example, new, nanosized contrast agents are allowing researchers to use conventional MRI technologies to determine whether breast or prostate cancer has metastasized to nearby lymph nodes - potentially making it possible to spare patients from biopsies and excision.
Imaging also is demonstrating its value as a molecular detective. Biopsies and focal gene/protein assays, while providing snapshot information about the cancer cell, may not capture the heterogeneous biological phenomena that occurs in the tumor. With imaging, however, we can more easily obtain serial information over time and better recognize this dynamic heterogeneity. We could track, for instance, how cancer cell phenotypes change in response to a drug over time. Such information will be used in clinical trials to document the molecular response to therapies, and will eventually be used by clinicians to determine whether and how treatment should be modified.
In addition to the excellent work of CIP, led by Dr. Dan Sullivan, in promoting the translation of imaging research to the clinic, MIP, led by Dr. Peter Choyke (see this week's Spotlight) is helping to move novel imaging probes, contrast agents, and imaging techniques into cancer clinical trials. NCI also is working on other fronts. The institute, for example, plays an important leadership role on the Interagency Council on Biomedical Imaging in Oncology, a group of federal health agencies and imaging technology developers, to expedite the delivery of new products to the market.
Imaging is influencing every area of cancer research. It's for that reason that imaging is one of the central components of the NCI National Advanced Technology Initiative for Cancer, or NATIc - an effort that, at its core, aims to deliver the advantages of technologies like imaging to the entire research community. The enthusiasm that surrounds this technology is well deserved, and I'm entirely confident that our investment in it will help to improve and save countless lives.
Dr. Andrew C. von Eschenbach
Director, National Cancer Institute
|
 |
 |

Attaching Beacons to Target Cells Results in Better Cancer Imaging
This is the second of a two-part series on cancer imaging. This week's article discusses how new contrast agents can better evaluate cancer processes in animal models.
Chemistry is king in the land of molecular imaging, says Dr. Peter Choyke, chief of the Molecular Imaging Program (MIP) in NCI's CCR. MIP's mission is to perfect and demonstrate a concept that could revolutionize diagnostic imaging.
This strategy delivers a molecular "beacon" to the outside of a cell to guide and enhance more precise imaging, Dr. Choyke explains. The concept is similar to the "Trojan horse" strategy that delivers chemotherapeutic agents, loaded inside immunoliposomes, to specific cancer cells (NCI Cancer Bulletin, April 12).
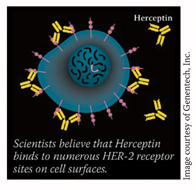
Targeted imaging probes are now being tested in animal models, Dr. Choyke notes. For example, by labeling the cancer drug trastuzumab, researchers can monitor it as it binds to the HER-2 protein receptor site. All tumors expressing the HER-2 protein can be imaged and emphasized with this imaging agent. The underlying principle may also apply to other cancers if binding sites can be found that distinguish those particular cells, he says. This system might not only dramatically improve diagnostic imaging of tumors, but also help monitor for recurrence and deliver therapeutic agents.
In the past year, Dr. Choyke's team has assembled a chemical arsenal of different nanoparticles, each designed to find binding sites on the surface of specific target cells. The key is to find a biological receptor that will distinguish a particular kind of cell - often a cancer cell in a particular organ - to the exclusion of all others, he says. Once delivered and locked onto their target, these designer molecules provide a beacon to image the cell's location.
Small groups of MIP scientists specialize in a particular imaging method, including magnetic resonance imaging (MRI), usually enhanced with a contrast agent; positron emission tomography (PET), which picks up positron-emitting radionuclides and can be combined with computed tomography (CT); and optical imaging.
The first stage in the development of an imaging agent involves synthesizing the agent to target a particular tumor or process, and then tweaking the imaging instruments to take advantage of it, Dr. Choyke explains. Then the agent can move to preclinical animal studies and to early-phase human clinical studies, he adds.
Dr. Martin Brechbiel, chief of NCI's Radioimmune and Inorganic Chemistry Section (RICS), and his team work closely with MIP. "The work of our labs is totally integrated," says Dr. Choyke, of the relationship. "There's a true synergy between RICS' chemistry and MIP's knowledge of imaging technology. Dr. Brechbiel's resources were already on the ground and primed, which has enabled us to develop and begin testing a number of compounds fairly quickly."
The innovation that distinguishes these programs' nanoparticle constructs is the chelate, or linking compound. "The delivery vector ligand binds to one location on the chelate," says Dr. Brechbiel. "On the other side, you chemically attach the imaging beacon you want to deliver to that particular target cell, wherever it might be in the body." The researchers must balance many characteristics, preserving the binding accuracy while enhancing the reliability and versatility of the delivery vector.
After an agent is produced and the imaging instrumentation is optimized, preclinical studies in mice allow a variety of cancer processes to be evaluated, says Dr. Choyke. For instance, MIP has projects looking at tumor angiogenesis and lymphangiogenesis, the latter of which, he adds, "is an understudied aspect of cancer metastasis because there has been no good way to image the lymphatics."
MIP's ultimate goal is to move these new imaging agents into clinical trials. "FDA's investigational new drug process represents an enormous hurdle," says Dr. Choyke. Even the largest drug companies are wary of the costs involved in developing imaging agents, estimated at about $250 million per agent. "But we're getting their interest with a tantalizing possibility," he says. "If you could use our system to help preselect which patients are more likely to respond to a treatment, you could conceivably run smaller trials with larger response rates."
By Addison Greenwood
|
 |
 |
Inhibition of Metastases in Preclinical Models of Osteosarcoma
Two NCI studies presented at the American Association for Cancer Research (AACR) annual meeting show the potential to decrease metastases in osteosarcoma by inhibiting an interaction or pathway involved in its spread.
Dr. Su Young Kim and colleagues in CCR's Pediatric Oncology Branch studied the effects of disrupting the interaction between the chemokine stromal-derived factor 1 (SDF-1) and its receptor, CXCR4, on lung metastases in a mouse model. SDF-1 and CXCR4 have been shown to be involved in the regulation of metastases of many human tumors, and CXCR4 expression in human osteosarcoma often correlates with poor prognosis.
The researchers collaborated with Chemokine Therapeutics of Vancouver, BC, and used their drug, CTCE-9908, a molecule that prevents CXCR4 from interacting with SDF-1. They saw a 67-percent decrease in the number of surface lung metastases in mice treated with CTCE-9908 compared with the controls.
"A previous phase I study of CTCE-9908 in healthy adults did not reveal any significant toxicities," comments Dr. Kim. "Combined with our current results, this suggests that clinical studies of CTCE-9908 in human osteosarcomas may be warranted."
In the second study, Dr. Xiaolin Wan and colleagues, also from the Pediatric Oncology Branch, examined the effects of the drug rapamycin on metastases mediated by the protein ezrin. The lab previously showed that ezrin expression is necessary for metastatic behavior in a mouse model of osteosarcoma.
The scientists discovered that the ezrin-related metastatic behavior was linked to an Akt-dependent mTOR pathway, the mammalian target of rapamycin. Blockading this pathway with rapamycin or its analog, cell cycle inhibitor-779 (CCI-779), led to significant inhibition of experimental lung metastasis in vivo, and prolonged survival of the treated mice. Whereas all 8 mice in the control group developed lung metastases, only 1 in 7 mice given rapamycin and 0 of 9 given the highest dose of CCI-779 developed lung metastases.
"These results suggest that blocking the mTOR pathway may be an appropriate target for strategies to reduce tumor cell metastasis," comments Dr. Wan. "However, we need to conduct more preclinical studies before we can begin to design clinical trials."
Novel MRI Approach Measures Early Tumor Response
A novel MRI approach reported at the AACR meeting may allow clinicians to assess an individual's response to a particular treatment with better accuracy and less toxicity than can currently be done.
Dr. Brian Ross of the University of Michigan used a technique called diffusional MRI to measure changes in the Brownian motion of water molecules within brain tumors before and after treatment with chemotherapy and/or radiation. The technique capitalizes on the fact that as cells die, their membranes break down, allowing greater movement of the water molecules that were once bound by them.
Cell death changes the diffusion of water within a tumor, allowing pretreatment measurements to be compared with those taken shortly after treatment. The researchers map their data onto a functional diffusion map (fDM), enabling them to visualize treatment-induced tumor changes, as well as regions not affected by treatment.
Dr. Ross reported on the results from 29 patients with malignant glioma. Not only could the researchers distinguish between stable and progressive disease at 3 weeks, the patients could also be successfully stratified, based upon the fDM measurement, into two populations based on their overall survival (18 months vs. 8 months).
"This study was the first series of clinical data on a single tumor type allowing for fDM to be clinically validated as a biomarker," said Dr. Ross. "Our results are completely independent of the field strength or manufacturer of the equipment used, allowing for easy testing in multicenter clinical trials."
Green Tea Compound Shows Prevention Prowess
Results from a small clinical trial presented last week at the AACR meeting provide strong evidence that a green tea compound may be able to prevent prostate cancer.
Italian researchers reported on a small, randomized, double-blind, placebo-controlled study testing whether EGCG, considered the most potent form of substances found in green tea leaves called catechins, could prevent prostate cancer in men at high risk for the disease. The study included 60 previously untreated men between 45 and 75 years old with high-grade prostatic intraepithelial neoplasia lesions. Approximately 25 to 35 percent of men with such lesions on prostate biopsy will have prostate cancer diagnosed within 1 year. Of the 30 men in the treatment arm, only 1 developed prostate cancer, compared with 9 of 30 in the placebo arm.
"This is quite impressive because the difference is highly significant," said study leader Dr. Saverio Bettuzzi. "Thirty patients in each arm is fine as a proof-of-principle study," he continued, while cautioning that many questions remain unanswered, including whether the treatment actually prevented cancer or simply delayed it.
Dr. Bettuzzi and colleagues, who will follow these trial participants for up to 5 years, hope to launch a larger trial that includes men from both Italy and the United States.
Potential Breast Cancer Drug Targets Tumors with BRCA Mutations
Scientists have discovered a drug that slows the growth of some breast tumors containing mutations in the genes BRCA1 and BRCA2, according to a study in mice reported in the April 14 Nature. BRCA mutations, which are usually inherited, account for only 5 to 10 percent of breast cancers, but women carrying them have up to an 80 percent chance of developing breast cancer by age 80.
The researchers found that giving the experimental drug to mice caused some tumors with BRCA mutations to become unstable and die. The drug inhibited an enzyme called PARP (poly [ADP-ribose] polymerase) that repairs DNA in tumor cells and is essential for the survival of tumors with BRCA mutations.
"These data in mice are exciting because they suggest that BRCA1- and BRCA2-associated cancers may be treated - and perhaps prevented - with an agent that targets a specific molecular pathway," says Dr. Sheila Prindiville of NCI's CCR. "The question now is whether the findings in mice translate to humans."
Chemopreventive Tamoxifen Use Rejected by Most Women At Risk
Among healthy women at high risk for breast cancer, only a small minority were willing to take tamoxifen as a chemopreventive agent to lower their risk for developing the disease, according to a study published April 11 online in Cancer.
The findings are based on interviews with 255 at-risk women, each of whom received a 15-minute educational session about tamoxifen's benefits and risks. "After the intervention, only 45 women (17.6 percent) said they were inclined to take tamoxifen," report researchers from the University of California, Davis.
Among those who rejected using tamoxifen preventively, many voiced concerns about adverse effects from the drug, especially risks for pulmonary embolism, painful intercourse, and cataracts. Most women said they were less concerned about tamoxifen's association with increased risks of endometrial cancer.
Rather than taking a drug to lower their risk, many women mentioned using other "self-preventive strategies," including breast exams, mammograms, and changes in diet and exercise, the researchers report.
|
 |
 |

Stem Cells and Cancer
PA-05-086
Application Receipt Dates:
May 10 and
Sept. 10, 2005;
Jan. 10, May 10, and Sept. 10, 2006;
Jan. 10, May 10, and Sept. 10, 2007;
Jan. 10, 2008
This funding opportunity will use the NIH R01 and R21 award mechanisms. For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2682. Inquiries: Dr. R. Allan Mufson - am214t@nih.gov; Dr. Jill Carrington - carringtonj@nia.nih.gov.
Understanding and Treating Tuberous Sclerosis Complex
PAS-05-085
Application Receipt Dates:
May 10 and
Sept. 10, 2005;
Jan. 10, May 10, and Sept. 10, 2006;
Jan. 10, May 10, and Sept. 10, 2007;
Jan. 10, 2008
This funding opportunity will use the NIH R01, R21, and R03 award mechanisms. For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2681. Inquiries: Dr. Mary Ellen Perry - mp372j@nih.gov.
Interactions between Stem and Progenitor Cells and the Microenvironment In Vivo
PAS-05-092
Application Receipt Dates:
May 10 and
Sept. 10, 2005;
Jan. 10, May 10, and Sept. 10, 2006;
Jan. 10, May 10, and Sept. 10, 2007;
Jan. 10, 2008
This is a reissue of PAS-03-172. This funding opportunity will use the NIH R01, R21, and R03 award mechanisms. For more information see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=2683. Inquiries: Dr. R. Allan Mufson - am214t@nih.gov.
For comprehensive information about NCI funding priorities and opportunities, go to http://www.cancer.gov/researchandfunding.
|
 |
 |

New Vaccine for Advanced Non-Small-Cell Lung Cancer
Name of the Trial
Phase I/II Study of Antitumor Vaccination Using alpha-1,3-Galactosyltransferase-Expressing Allogeneic Tumor Cells (HyperAcute Lung Cancer Vaccine) in Patients with Advanced, Refractory, or Recurrent Non-Small-Cell Lung Cancer (NCI-04-C0049). See the protocol summary at http://cancer.gov/clinicaltrials/NCI-04-C-0049.
 Principal Investigator
Principal Investigator
Dr. John C. Morris, NCI Center for Cancer Research
Why Is This Trial Important?
Lung cancer is the leading cause of cancer-related death in the United States, with more than 163,000 people expected to die from the disease in 2005.
Lung cancer is most often diagnosed at advanced stages when it is difficult to treat. About 80 percent of non-small-cell lung cancer (NSCLC) cases are detected when they have progressed to stages III or IV, and life expectancy ranges from 6 to 12 months.
Researchers are testing a vaccine intended to stimulate the immune systems of NSCLC patients to attack their tumors. The vaccine consists of killed human NSCLC cells that have been genetically altered to express a nonhuman carbohydrate on their surface. This carbohydrate, known as alpha-Gal, is present in lower animals, but not in humans. Alpha-Gal is a powerful antigen that causes a rapid, hyperacute antibody response whenever foreign tissues bearing it are introduced into the human body. The response is powerful enough to destroy transplanted cells and tissues within hours.
Athough cells making up naturally occuring NSCLC tumors in patients do not express alpha-Gal, they share other cell-surface molecules with the genetically altered NSCLC cells introduced by the vaccine. The researchers hope those similarities will allow the antibodies and immune cells targeting alpha-Gal to redirect their attack and destroy patients' own tumor cells.
"Until this trial, this type of vaccine had never been tested in patients," said Dr. Morris. "If it works, it may lead to tumor shrinkage or disease stabilization."
Who Can Join This Trial?
The researchers will recruit 52 patients aged 18 and over who have been diagnosed with advanced NSCLC. See the list of eligibility criteria at http://cancer.gov/clinicaltrials/NCI-04-C-0049.
Where Is This Trial Taking Place?
The study is taking place at the National Institutes of Health Clinical Center in Bethesda, Md.
Contact Information
For more information, contact the NCI Clinical Studies Support Center at 1-888-NCI-1937. The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at
http://cancer.gov/clinicaltrials/ft-all-featured-trials.
|
 |
 |

FDA Clarifies Submission Requirements for "Exploratory" IND Studies
In a new draft guidance published for comment in the April 14 Federal Register, the Food and Drug Administration (FDA) clarifies submission requirements for early phase I exploratory studies under an investigational new drug (IND) application.
FDA says such applications often contain more data than required by regulations. According to the new draft guidance, "Exploratory IND Studies," depending on the study, the preclinical testing programs for exploratory INDs "can be less detailed and more flexible than for traditional INDs."
FDA developed the exploratory IND guidance document with input from NCI through the Interagency Oncology Task Force (IOTF), which fosters closer cooperation between the agencies. "We were looking for ways to streamline the early drug development process," notes Dr. Michaele Christian, associate director of NCI's Cancer Therapy Evaluation Program and IOTF member. The goal is to allow researchers to conduct early proof-of-principle evaluations of compounds and target effects to identify promising candidates for more extensive testing, she adds.
Dr. Joseph Tomaszewski, chief of NCI's Toxicology and Pharmacology Branch and acting associate director of the Developmental Therapeutics Program concurs, saying, "It will be especially beneficial when you have a series of analogs and you cannot or do not want to make a decision about which one agent to move forward with. This guidance will allow investigators to test each one in a limited clinical trial that will provide sufficient human data to help inform the decision."
FDA is seeking input on the draft guidance, and NCI is especially encouraging comment from the cancer research community during the 90-day comment period. The document can be viewed and downloaded at http://www.fda.gov/cder/guidance/6384dft.pdf.
Although FDA's regulations are flexible, many researchers and drug sponsors "have not taken full advantage of that flexibility and limited, early phase I studies - such as those described in this guidance - are often supported by a more extensive preclinical database than is needed for those studies," FDA contends.
The draft guidance defines an exploratory IND study as a clinical trial that occurs very early in phase 1, involves very limited human exposure to a product (e.g., maximum 7 days), and has no therapeutic intent. Such tests are useful, for example, to "select the most promising lead product from a group of candidates designed to interact with a particular therapeutic target in humans," FDA notes. Exploratory testing usually occurs prior to the more extensive "traditional dose escalation, safety, and tolerance studies" conducted later in phase 1 development of drugs and biological products.
|
 |
 |
NCI/HHMI Translational Research Training Meeting
NCI and the Howard Hughes Medical Institute (HHMI) will sponsor the "Translational Research Training Meeting: A Cancer Perspective," June 16-17, 2005, at the Natcher Conference Center on the NIH Campus in Bethesda, Md. The program will include the 2004-2005 Annual Report of the President's Cancer Panel on Translating Research into Cancer Care: Delivering on the Promise, as well as presentations of successful, established translational research training programs, a session on future training needs in academia and industry, and a comparison of translational research training for M.D.s, M.D./Ph.D.s, and Ph.D.s.
This meeting is intended to reveal more effective approaches to training physician-scientists and basic researchers to bridge the gap between basic science and its application to medicine. The comments and discussion generated will be taken into account as NCI plans and implements future training programs. HHMI is committed to enhancing the training of M.D. and Ph.D. biomedical scientists to facilitate the attainment, translation, and transfer of biological knowledge between laboratory, clinical, and public health practices.
Registration is free, but space is limited; early registration is encouraged. Go to https://cms.palladianpartners.com/cms/1108413029 for agenda and registration information.
Smokers Needed to Evaluate NCI Cessation Web Site
Federally employed smokers who are interested in quitting and have not yet enrolled in a related program are invited to visit http://www.smokefree.gov/eval to see if they are eligible to use and evaluate a smoking cessation Web site created by NCI and its partners.
Smokefree.gov is an online tool that offers evidence-based information to smokers who are ready to quit. Input from current smokers about the site will not only provide valuable information to improve the site, but also will be of service to those who may be seeking information about quitting smoking. Federal employees who smoke and who visit the site will be invited to evaluate the site and its features, and provide feedback on the usefulness of its self-help guides and other tools.
GvHD Conference Slated for June
On June 6, NCI; the National Heart, Lung, and Blood Institute; NIH's Office of Rare Diseases; the Health Resources and Services Administration; and the Department of Defense will cosponsor a 1-day conference, "Chronic GvHD: The Next Frontier in Transplantation Research." The meeting is part of the NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease and will take place in Bethesda, Md. The registration deadline is May 1; for additional information, go to http://www.palladianpartners.com/gvhd.
Symposium on Skeletal Complications of Malignancy
NCI, the Paget Foundation, and the University of Virginia Health System are sponsoring a symposium on skeletal complications of malignancy, April 28-30, at the Natcher Conference Center on the NIH campus in Bethesda, Md. For more information and to register, go to http://www.paget.org/conferences.asp.
|
 |
 |

University of Pittsburgh Cancer Institute
Director: Dr. Ronald B. Herberman • 5150 Centre Avenue, Pittsburgh, PA 15232
Phone: 412-647-2811 • Web site: http://www.upci.upmc.edu
 |
 |
Featured Meetings and Events |
 |
|
A comprehensive calendar of cancer-related scientific meetings and events sponsored by NCI and other scientific organizations, is available at: http://calendar.cancer.gov/
|
|
Background
The University of Pittsburgh Cancer Institute (UPCI) was founded in 1984 through a collaboration between the University of Pittsburgh Medical Center (UPMC) and its affiliated hospitals, the University of Pittsburgh, and Carnegie Mellon University. UPCI was originally known as the Pittsburgh Cancer Institute. Dr. Ronald B. Herberman, former chief of the NCI Biological Therapeutics Branch, left NCI to establish UPCI and remains as its founding director. UPCI was designated an NCI Comprehensive Cancer Center in 1990 and, over the next decade, expanded to include a collaborative network, the UPMC affiliated Cancer Centers, with 43 locations throughout western Pennsylvania and adjacent regions of Ohio and West Virginia. Today, the network serves that region's population of 6 million through a "hub and satellite" administrative structure, with UPCI's Hillman Cancer Center as the flagship facility and home to more than 500 faculty and staff who represent approximately 30 medical disciplines.
 Clinical Specialties
Clinical Specialties
While UPCI offers treatment for a wide range of cancers, the institute has gained international recognition for its treatment of advanced melanoma, as well as brain, head and neck, prostate, lung, ovarian, and breast cancers. UPCI participates in several multi-institutional clinical research alliances, including the Eastern Cooperative Oncology Group, the Radiation Therapy Oncology Group, the North American Brain Tumor Consortium, and the American College of Surgeons Oncology Group.
Research Activities
In addition to sponsoring basic research on cancer, UPCI is currently running more than 350 clinical trials in cancer prevention, diagnosis, and treatment. UPCI also sponsors research programs that focus on cancer control in the context of populations and social interaction. The Behavioral Medicine and Oncology Program, for example, is studying how behavioral factors can influence the biology of cancer and the well-being of cancer patients, while the Cancer Epidemiology, Prevention and Control Program is examining environmental and physiological factors that influence cancer susceptibility within populations by working in conjunction with a newly-established Center for Environmental Oncology.
Other Notable Programs
UPCI makes the latest and most promising radiation technologies available to its patients through the UPMC Cancer Centers, which serve 36,000 new patients each year, as well as through partnerships with private companies that license supporting technologies. This arrangement has brought intensity-modulated radiation therapy, for example - a relatively new technology that targets radiation therapy to tumors while sparing healthy tissue - to patients in their local communities. Using telemedicine, experts at the institute's main campus can monitor patients and train clinicians in hospitals throughout the region that UPCI serves.
|
 |
|