Investigation: # 01MI061 and 01MI063
Two Individuals Overcome By Hydrogen Sulfide Gas While Cleaning a Farm Facility Water Well With 28% Liquid Muriatic Acid
SUMMARY
 |
RECOMMENDATIONS
INTRODUCTION
On August 15, 2001, two males, a farmer, 42 years old, and a friend, 60 years old, died from inhalation of hydrogen sulfide gas while cleaning the well screen of a driven point well with 28% uninhibited liquid muriatic acid. On August 21, 2001, Michigan Fatality Assessment and Control Evaluation (MIFACE) investigators were informed by the Michigan Occupational Safety and Health Act (MIOSHA) 24-hour fatality report system that a work-related fatal injury occurred on August 15, 2001. On October 4, 2001, MIFACE researchers went to the farm location and interviewed a family member familiar with the incident as well as the emergency responder. The death certificate, autopsy results, and police reports were obtained during the course of the investigation.
MIOSHA did not conduct an on-site investigation of this incident because they had no jurisdiction; there was no employer/employee relationship. The responding police consulted with a MIOSHA compliance officer to gather information for the police report of the incident.
INVESTIGATION
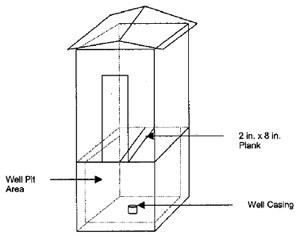 |
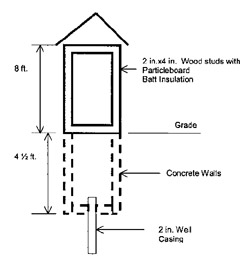
The family member told MIFACE that the well involved in this incident was a driven point well. A driven point well is constructed by driving assembled lengths of pipe into the ground. A drivepoint is a cast, hollow metallic cylinder with a reinforced point. Above the drive point is another piece of piping containing 2-3 ft. of screen. Subsequent pieces of pipe are added while driving the drivepoint to the aquifer. The family member did not know the depth of this well. On average, driven point wells are usually less than 30-50 ft. deep and 2 in. or less in diameter. A driven point well is considered a shallow well drawing its water from the groundwater nearest to the land surface. The condition of the various components of the well, such as the metal well pipe, well screen, well cap and pump are unknown. The screen size and type are also unknowns.
The age of the well is unknown. The location of the septic tank is also unknown, however this well was within the animal housing/outside confinement area. When the MIFACE investigators visited the farm, the well had been capped and covered with a large pile of dirt.
The well owner used an over–the-counter grade of 28% muriatic acid to clean the well screen. The emergency responders found an empty 64-ounce container of muriatic acid, a ¼ full 64-ounce container of muriatic acid, funnel and ½ in. diameter white polypropylene plastic tubing. The funnel was outside of the well shed; the tubing was inside of the well. The well casing was open. The wind was blowing from the northwest at approximately 10 miles per hour, according to the emergency responder report.
According to the family member, the well was running “slow” and the owner thought the problem could be a plugged or incrusted well screen. The well screen is located at a point directly above the drive point and allows water to flow into the well, stopping the uptake of sand. The decline of the amount of water flowing from the well could have been caused by many factors, such as well hydraulic change, water chemistry and microbiology change, depletion of the aquifer (the area had experienced prolonged drought conditions) and blockage of the well screen. A chemical, biological or physical (or a combination of) can also cause blockages at a well screen that can decrease water yield from the well. Chemical blockage is when the groundwater conditions deposit minerals (hard water) that build up scale or a mineral deposit on the screen. Biological blockage is due to naturally occurring slime forming bacteria, iron reducing bacteria and sulfate reducing bacteria found in the water. Physical blockage can be a flow path blockage caused by an accumulation of clay and silt in the screen as a result of over pumping, poor initial well development, wrong type of screen or poor screen placement.
 |
| Figure 1 |
Liquid muriatic acid is easily available and is inexpensive, therefore homeowners frequently use it to clean well screen incrustation. Uninhibited muriatic acid can cause destruction of the well screen and pitting of the drop pipe. The liquid muriatic acid the victim was using did not contain any inhibitors. See Figure 1 for an example of the product used by the victims.
Based on the position of the equipment being used to clean the screen, it appears that the muriatic acid was being poured into the funnel attached to the plastic tubing placed in the well outside of the protective structure. It is not known if both victims were outside during this process, or if the well owner was inside the structure while the assistant poured the acid from the outside (or vice versa).
Hydrogen sulfide gas can occur naturally in groundwater. The water may have a decomposing underground deposit of organic matter and/or contain sulfur-reducing bacteria. Sulfur reducing bacteria break down sulfur compounds, such as naturally occurring sulfates, and produce hydrogen sulfide gas. Hydrogen sulfide could also have been formed by the addition of the muriatic acid to the well. When an acid is added to water, free hydrogen ions are released. If iron sulfide is present in the well water, the free hydrogen will react with the iron sulfide producing hydrogen sulfide.
During the well cleaning process, as the muriatic acid was added to the well, there may have been a “blowout”, resulting in a hydrogen sulfide emission from the well. A blowout is the movement of the acid and well water mixture back to the surface through the piping. This can occur when an acid is rapidly added to a well and a large quantity of carbon dioxide is produced. Hydrogen sulfide, as well as carbon dioxide would have been emitted from the well during the blowout. Both hydrogen sulfide and carbon dioxide are heavier than air, and would settle into the well pit. The protective structure also contained the gases and didn’t allow for any movement of the gases by the wind.
MIFACE postulates that a blow-out occurred because of the presence of the acid/water mixture at the base of the well pit. It is unknown if the presence of the hydrogen sulfide was naturally occurring, was a product of a hydrogen-iron sulfide reaction, or was a combination of both. The hydrogen sulfide was released into a confined area (at the well pit inside the building). It is unknown if the victims were inside of the protective structure when the blowout occurred or whether they entered later.
The severity of the physical effects of exposure to hydrogen sulfide increase when the hydrogen sulfide concentration in the air increases. At very low levels in air (0.13 parts per million), hydrogen sulfide gas smells like "rotten eggs" and a person can breathe the air a long time. At higher concentrations (100-250 parts per million), hydrogen sulfide deadens a person’s sense of smell and stings the eyes and throat. At 600 parts per million, death can occur within 2 minutes, and at higher levels, a person can become unconscious and die quickly.
A family member of the victim detected the muriatic acid odor and went to investigate. The family member noticed the individuals and went to the house to call emergency personnel. When the emergency responders arrived, the individuals were found in the well pit in approximately 4 in. of water/muriatic acid solution. Emergency personnel could detect a muriatic acid odor from the road. One of the individuals died at the scene, the other individual was transported to a local hospital, where he later died.
The emergency responder later noted damage had occurred to his clothing and the clothing worn by others. The emergency responders wore protective boots and respirators, but wore everyday clothing. The water/acid mixture damaged the pant legs of the responders entering the well pit.
A water sample from the well involved in this incident could not be obtained to check for sulfates or other contaminants due to the closing of the well prior to the MIFACE visit. Another water sample from a well within 300 ft. of the well associated with the fatalities was obtained to determine general water conditions for the area. The U.S. Department of Interior and the Water Quality Association classify water hardness. Hardness is caused by compounds of calcium and magnesium and a variety of other metals dissolved in the water. The water sample from the new well was tested for hardness and iron. The water had 20-grains/gallon hardness and iron was not present in the water. Based on the results of the water test, the well water from the new well would be considered "very hard". Note: It is unknown if the same water aquifer was being tested.
CAUSE OF DEATH
The medical examiner recorded the cause of death as asphyxia as a consequence of hydrogen sulfide poisoning for each of the victims. The medical examiner cited in the medical examiner report the hydrogen sulfide level in the blood of each of the victims; based on the amount of hydrogen sulfide in the blood of the victims, and comparing the level to other accidental fatal exposures from hydrogen sulfide, the medical examiner concluded that the hydrogen sulfide exposure was the cause of death.
RECOMMENDATIONS/DISCUSSION
• Private water well owners should not use uninhibited, liquid muriatic acid to clean well screens; owners should explore acid alternatives that are in granular or palletized form, slower dissolving, produce less hazardous fumes and are more environmentally friendly.
There are many acids or other products on the market that are available in the granular or palletized form. These acids and/or other products are available in slow dissolving pellets, which can usually be poured into the well pipe at the priming-plug port located prior to the pump inlet without disconnecting the pump. This form of acid is safer to use. There are different types of acids available to clean well screens: muriatic, sulfamic, phosphoric, glycolic as well as combinations of acids. Well owners should consult an expert for advice.
Factors that should be considered before cleaning the well screen should include the type of acid to treat the type of incrustation on the screen, the volume of acid solution to use, personal safety equipment, and surface equipment for mixing the acid. An estimate of the volume of acid needed for cleaning the well screen of the incident well was between ¼ and ½ cup; the victims used nearly 2 gallons of acid.
Over-the-counter liquid muriatic acid is not recommended for well cleaning by non-professionals. Liquid muriatic acid may not contain inhibitors, increases the potential of blowouts, and can expose both people and the environment to unsafe conditions. If liquid muriatic acid is the only product that can be used to dissolve the incrustation on the well screen, a licensed well contractor should perform the cleaning.
• Well owners should consider well location, well construction, and well management and maintenance issues so the well and water quality are protected from outside contamination sources.
Well Location
MIFACE cannot determine definitively if the well water had been contaminated by an outside source. The driven point well was located downhill from a cattle barn with a dirt floor. The cattle barn was a potential source of surface water contamination. The farmer’s shallow well, located downhill from the livestock barn or failing septic system runs a greater risk of contamination than a well on the uphill side from these pollution sources. Decomposing manure can give off hydrogen sulfide, as well as add bacterial contamination to the water. The well pit would collect any runoff from the barn. In shallow aquifers, groundwater often flows in the same direction as surface water. EPA recommends that the well should be placed at least 50-100 ft. from an animal shelter/yard without a concrete floor.
Well Construction
The items of concern involving well construction are: height of the piping above the ground, well condition, well age, and well depth. The family member could not give any information about the items listed above, so it is unknown if well construction was a factor in this incident. On larger diameter wells, the well cap should be installed to prevent leakage into the piping and should have a screened vent. The age of a well may affect both the condition of the piping as well as the location. Piping may become corroded with age. Often, a well that was located away from farming activities years ago is now in the middle of the farmstead and surrounded by potential contamination sources.
Well Management and Maintenance
Well water should be tested annually to determine if there are any changes in the water chemistry or other contaminants. Determining the water constituents will help identify incrustation problems and potential preventative measures such as well screen selection. Well screens should be selected to let water enter the well at no more than 1 foot per second, and keep sand out of the well. Screen materials should be selected based on the water characteristics and type of cleaning agents that could be used based on the water constituents. Incrustation of the screen can occur where the water passes through the screen where there is a sudden drop in water pressure during pumping.5 Keeping the pressure drop through the well to a minimum by having the largest possible area of openings into the well and by keeping the pumping rate down will reduce the potential for incrustation. If water flows too quickly through the screen, a large pressure drop occurs, and the probability of incrustation occurring increases.
To protect the wellhead, owners should build up the soil around the wellhead or install cement curbs to divert runoff water.
Owners should contact a qualified well driller or pump installer prior to commencing any maintenance work on the well.
REFERENCES
MIFACE (Michigan Fatality and Control Evaluation), Michigan State University (MSU) Occupational & Environmental Medicine, 117 West Fee Hall, East Lansing, Michigan 48824-1315. This information is for educational purposes only. This MIFACE report becomes public property upon publication and may be printed verbatim with credit to MSU. Reprinting cannot be used to endorse or advertise a commercial product or company. All rights reserved. MSU is an affirmative-action, equal opportunity employer.
To contact Michigan State FACE program personnel regarding State-based FACE reports, please use information listed on the Contact Sheet on the NIOSH FACE web site Please contact In-house FACE program personnel regarding In-house FACE reports and to gain assistance when State-FACE program personnel cannot be reached.