|
Under the direction of Michael Shelby, Ph.D., Director, CERHR at NIEHS, scientific and support staff at NIEHS and Sciences International, Inc. operate the Center for the Evaluation of Risks to Human Reproduction (CERHR). The Principal Investigator, Anthony Scialli, M.D., leads the scientific and support staff at Sciences International, Inc.
Center Organization
Committees
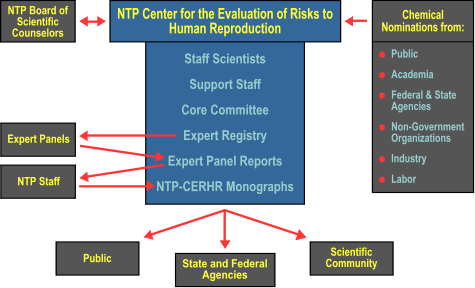
The NTP Board of Scientific Counselors provides oversight for CERHR and offers advice on priorities, directions, and the adequacy of the Center's process.
The Core Committee is a an advisory body consisting of scientists who represent government agencies represented on the NTP Executive Committee. The current committee members are: Dr. Jennifer Seed, US Environmental Protection Agency, National Center for Environmental Assessment; Dr. Elizabeth Whelan, National Institute of Occupational Safety and Health, Centers for Disease Control and Prevention; Dr. David Morse, US Food and Drug Administration; Dr. Marilyn Wind, US Consumer Product Safety Commission; Dr. Adolfo Correa, National Center for Environmental Health, Centers for Disease Control and Prevention, and Dr. Michael Shelby, National Institute of Environmental Health Sciences.
The Core Committee's duties are:
- To review chemicals nominated for evaluation;
- To recommend chemicals to be evaluated;
- To approve members to the CERHR Expert Registry;
- To recommend Expert Panel members for approval by the Director, NTP and
- To review Expert Panel Reports.
Expert Panels are the independent working groups of scientists that produce the individual reports. The Panels are comprised of individuals with expertise in one or several scientific areas integral to a chemical evaluation. These scientists are selected from a registry built and maintained by CERHR staff and represent academia, industry, and government research and regulatory agencies. Suggestions for additional scientists for this Registry may be sent to CERHR accompanied by a description of expertise and a curriculum vitae. The Panel's purpose is:
- To critically review all data relevant to understanding a chemical's potential to cause developmental or reproductive toxicity in humans;
- To reach a scientifically-sound and informative assessment of the potential for adverse reproductive effects associated with exposure and
- To produce an Expert Panel Report.
CERHR Expert Panel Guidelines
|