You are here:
Funding
- Overview
- Funding Opportunities
- Funding Process
- Funded Research
- Clinical Research
- Policies & Guidelines

Osteoarthritis Initiative
Updated June 24, 1999 (historical)
Summary of Epidemiology/Biostatistics/Genetics Subcommittee Discussions
Subcommittee participants (affiliations):
David Felson (Boston University)
Claire Francomano (National Human Genome Research Institute)
Rosemarie Hirsch, alternate chair (National Center for Health Statistics)
Marc Hochberg (University of Maryland-Baltimore)1
Kent Johnson (Center for Drug Evaluation and Research, U.S. Food and Drug Administration)
Richard Leff (Bayer Corporation)
Markus Hosang (Hoffman LaRoche)
Stefan Lohmander, chair (Lund University)2
Joan McGowan (National Institute of Arthritis and Musculoskeletal and Skin Diseases)
Leena Sharma, scribe (Northwestern University)
_____________
1representing the American College of Rheumatology
2representing the Osteoarthritis Research Society International
The initial April '99 exploratory meeting was headlined 'National Institutes of Health - A public-private partnership for the development of biomarkers and surrogate endpoints for osteoarthritis'. The document continued '.to design, develop, and support a research project to identify, test, and validate biomarkers as surrogate endpoints for clinical trials in OA'. Biomarkers in this particular context are defined as novel imaging technologies and soluble protein factors. It is further assumed, for the purpose of this draft, that the clinical trials will include the claim of demonstrating delay or prevention of progression of joint damage (structural progression).
Focus questions for consideration include:
-
What are the central (and auxiliary) hypotheses to be tested?
-
What risk factors, diagnostic criteria and structure can be used to develop useful cohorts?
-
Are marker findings identified in high-risk groups generalizable to 'garden variety' forms of OA?
-
Do existing population studies and clinical trials provide sources of relevant information, or starting points for expansion?
-
Can genetic markers contribute to identification of high-risk groups or provide other insights?
-
What statistical considerations should be addressed?
-
What data/samples/x-ray/MRI, etc. should be collected in the course of a prospective study?
Useful study cohorts - Subcommittee discussions focused on questions of cohort selection and criteria. It was recognized that OA is multifactorial in pathogenesis and heterogeneous in presentation and progression. In general, progression is slow and extends over years, with a long asymptomatic phase. Two complementary avenues for cohort selection were explored and their respective pro's and con's discussed. Further discussion of this issue will be needed.
Large population-based cohort - Would have advantages in e.g. increasing odds for being representative and generalizable to population as a whole. Concerns include size of cohort which may need to be very large to include enough high-risk individuals, OA cases and progressors relevant for putative treatment. Basic approach would be to use multicenter cohorts, subsample those at high risk or have early disease, and evaluate all the subjects the same way.
Smaller focused cohorts - The selection of these cohorts would be based on specific risk factors, disease stages, joints, etc. The selection criteria for these cohorts would be concordant with criteria for patients that may be included in current and future clinical trials to prove delay or prevention of structural progression. Ultimately, they would also be the target groups for the putative treatment. Again, all subjects would be evaluated the same way in this multicenter approach. Concerns would include whether selection would ensure representative and generalizable results.
Points discussed - It was considered by some members of the committee that since the key goal of this initiative is to develop surrogate markers of OA to facilitate disease modifying intervention and trials, the cohort(s) should be composed of diseased subjects with symptomatic OA, which would currently correspond to group (A) in Figure 1. If the target population for trials and treatment is expanded to subjects at high risk for developing symptomatic OA, the cohorts could include groups (B), (C) and (D), dependent on proposed claims for putative treatment. This approach was argued to maximize power and focus resources. It was further commented that group (A) includes all diseased subjects in population, but that only a subset seeks care. Other members pointed out that a large enough population-based study (E) would incorporate all subsets. A population-based study would have the potential to address additional and broader relevant issues. Clearly, these different designs are not mutually exclusive, but may be complementary.
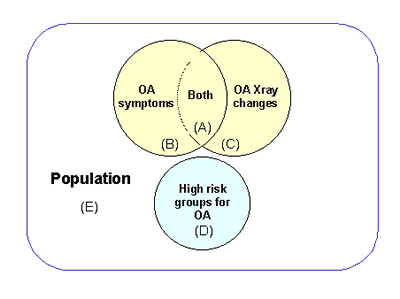
Figure 1. Schematic to illustrate relationship between population-at-large
and different cohorts. Areas not intended to represent relative size of groups.For all approaches, a further development of specific questions and hypotheses will be needed to adequately project the needed size of the studies.
Examples of specific cohorts mentioned for possible inclusion in study include: (1) families with Mendelian disorders, (2) cohort with early structural disease, (3) those with symptoms but without structural disease [no radiographic OA at baseline, group (B) in Figure 1], (4) cohort with established risk factors [e.g. meniscectomy would offer advantage of knowing day 0 as opposed to studies which identify onset of disease by symptom onset], (5) older women, (6) heavy women, (7) childhood hip disease, (8) multicenter community recruited cohort(s) with radiographic and symptomatic OA at baseline [to maximize the n of the relevant group of OA patients, i.e. those in whom disease modifying therapy would be considered, group (A) in Figure 1], (9) a polyarticular cohort, (10) genetically "pure" populations (e.g. Amish, Icelanders).
It was not clear from the discussions if the group favored a single joint approach, or different joints studied in different subsets, or a true 'polyarticular' approach.
A concern for any approach is that optimal biomarkers may well be different for different OA subsets, different disease stages, different joints, etc. Biomarkers may be different for definition and study of early-stage high-risk groups and disease progression, and for study of established (late-stage) disease groups with a combination of symptoms and structural change, Figure 2. The time lag between disease initiation and onset of overt disease is likely years, and is variable. Not all individuals, even in high risk groups, develop overt disease as currently defined. Progression is intermittent and overall rate variable. It was remarked that imaging biomarkers and circulating biomarkers of OA represent different dimensions of the disease, and it is not clear which one should be regarded as the gold standard, if any.

Figure 2. Schematic to illustrate slow, variable rate
of OA progression, and delayed onset of overt diseaseUtility of current studies and clinical trials - Several ongoing studies and trials could yield materials and information relevant to the goals of the initiative. There is, however, a concern that some of the previous studies have used unreliable and inaccurate methods with regard to collection of data, radiographs, and samples. A review of existing potential sources of relevant information and samples was strongly encouraged, with a view to analyzing the utility of each material. It was suggested that NIH commission such a review before the next meeting. This review should include past, ongoing and planned OA clinical trials from which relevant samples and data may be generated. In the pilot phase of the initiative, these cohorts could yield important information for hypothesis generation.
Existing cohorts include the following
US based cohorts: (1) Baltimore Longitudinal study of aging, (2) Framingham OA Study, (3) Study of Osteoporotic Fractures, (4) Michigan Bone Health Study, (5) Johnston County OA Study, (6) NHANES, (7) Indiana University OA Study, (9) Northwestern University OA Study, (10) Health ABC Study; and the following Europe based cohorts: (11) Zoetemeer Study, (12) Chingford OA Study, (13) Rotterdam OA study, (14) Bristol Knee OA Study, (15) Lund Posttrauma Knee OA Study, (16) Ulm OA Study, (17) Göteborg Study of 70-year olds, (18) Spenshult Knee OA study.
In addition, several ongoing clinical trials of OA have collected relevant data and samples. These may yield important information, since baseline data and samples have been collected. Trial cohorts could be followed prospectively, following the conclusion of the trial.
Finally, several large multicenter studies to screen for OA genes are being initiated or are under way. Since these studies intend to collect much the same baseline information, radiographs and samples which are considered in this initiative, avenues for interaction should be explored. Even if the genetic studies per se do not plan extensive follow-up, cohorts could be continued under a different umbrella. They would have the advantage that genetic information may be available.Statistical considerations - It was recognized that this is a central aspect of study planning and that biostatistics expertise will need to be part of the initiative early on. The subcommittee thus strongly encouraged identification and recruitment of the relevant experts in the continued work.
Data and samples to be collected - This will need to include demographic, clinical and comorbidity data, imaging data, samples of body fluids, and DNA. This issue will need considerable further development, and standardization of collection methodology is critical to a multicenter study of this kind. An illustration of the balance between the 'lean and mean' and the 'with everything' approach is given with Figure 3. All samples collected will need to be accompanied by a certain minimum of study-critical information (central bull's eye). For some samples, centers, or questions, additional information may be collected (represented by outer rings). A careful assessment of the appropriate balance between required and useful information will be required.
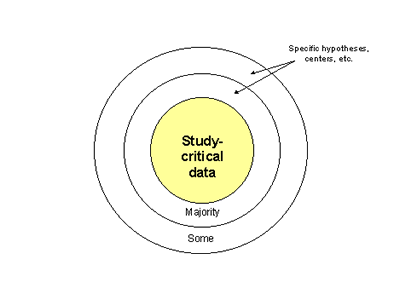
Figure 3. Schematic to illustrate possible
organization of data and sample collection.



