
 1663
1663
|
|

SpotlightPavlov's Bees, From Fungus Food to Biofuel, A Cure for the Brittle Diamond, Out on a WHIMPavlov's BeesTake a little behavioral psychology, add sugar water, and what have you got? Trained bees. Bomb-sniffing bees. In Pavlovian tradition, members of the Laboratory's Stealthy Insect Sensor project have used sweet treats to condition honeybees to respond to the chemical scent of explosives. The bees extend their proboscis (tongue) when exposed to explosives. Team members have trained the bees to do this by rewarding them with a dose of sugar each time they react to the smell of explosive chemicals such as TNT and C-4. Proboscis extension is a reflex normally triggered by the presence of nectar. Like dogs, which usually get the scent-detection assignments, not all bees respond well to training, but the team has improved the odds by adding a memory enhancer to the sugary reward. "I can't tell you the exact mix of this ‘cocktail,'" project member Kirsten McCabe apologizes. "We're hoping to patent it." What she's willing to say is that it's made of enzymes that play an essential role in memory formation and that it includes that universal waker-upper, caffeine. The trained bees ride five at a time in a toaster-size box, the Proboscis Extension Reflex Platform (PERP), which can be hand carried or mounted on a robot. The PERP concentrates scents and contains a camera that watches the insects. "A software imaging program captures what the camera sees," says McCabe, "and signals a hit through an image on a monitor or an auditory tone." The tone can be turned off in situations requiring stealth. Why use bees instead of dogs? They train faster, are less conspicuous, are cheaper to maintain, can work longer hours, and once inside their carrier, require no special handler. And they pick up scents at parts-per-trillion concentrations. The bee system will be validated this summer in field trials with the Laboratory's protection force subcontractor, SOC LA. And it'll be featured on the Discovery channel series, "Next World," in an episode titled "Security in the Future," tentatively scheduled for September 5. Besides McCabe, members of the bee team are Robert Wingo, Rhonda Robinson, and Sherri Sherwood, all drawn from the Laboratory's Bioscience, Computer, and Environmental Protection Divisions. Tim Haarmann, a Bioscience researcher, is a consultant. From Fungus Food to BiofuelThe fungus Trichoderma reesei is exceptionally talented at getting the sugary food it needs, and the Laboratory's Diego Martinez thinks that skill could be put to good use making biofuel. T. reesei makes enzymes, such as cellulases, that turn plant fibers into simple sugars. "It's actually the world's record holder for enzyme production," says Martinez. Those enzymes might be the economical mechanism for turning plant material (biomass) into the sugars that are fermented into burnable fuel. Current conversion methods are too costly for potential biofuel sources such as switchgrass, wood from fast-growing trees, and agricultural crop residues. 
It's not a squid. It's a fluorescence microscopy image of T. reesei, stretching out its growth filaments. Image credit: Mari Valkonen, VTT Technical Research Centre, Finland
Martinez, a bioscience graduate student who splits his time between Los Alamos and the University of New Mexico, is part of a research team led by the Department of Energy's Joint Genome Institute and Los Alamos National Laboratory. The team has decoded T. reesei's genetic sequence, looking for ways to enlist the fungus in the production of biofuels. What team members learned about T. reesei was unexpected. For such a prolific enzyme producer, the fungus makes only a surprisingly limited variety of cellulases. But it still outstrips other fungi in overall production. How can that be? "T. reesei's enzyme-encoding genes are organized for efficiency," says Martinez, who led the analysis of the genome data. "They're in clusters instead of being scattered through the genome. An entire cluster is activated when the fungus encounters food, so all the genes go to work at the same time." Martinez thinks scientists may now be able to supplement T. reesei's limited-but-voluminous enzyme supply to create an improved enzyme "brew" for breaking down plant cells. They can either put additional enzymes into the mix or add genes to T. reesei's genome so the fungus can produce more enzyme types. Other Laboratory researchers involved in the work are Thomas Brettin, David Bruce, Chris Detter, Cheryl Kuske, Olga Chertkov, Melissa Jackson, Cliff Han, Monica Misra, Nina Thayer, Ravi Barbote, and Gary Xie. A Cure for the Brittle DiamondA diamond is a paradox. It's the hardest naturally occurring substance; you can't scratch it except with another diamond. But it's also brittle and prone to fracture. It can break if struck sharply enough or exposed to thermal extremes. The diamonds we wear typically escape that fate, but industrial diamonds aren't so lucky. As essential components of drill bits and other cutting tools, they undergo serious punishment in the form of friction, impact, and high heat. As a result, they wear out, and so do the tools made with them. 
A roller-cone drill bit equipped with teeth made of the Laboratory's newly licensed diamond composite.
U.S. Synthetic, a leading producer of diamond-enhanced cutting tools, has found an answer for the problem in a technology just licensed from Los Alamos National Laboratory: a composite of diamond (a crystalline form of carbon) and silicon carbide. This superhard material was developed at the Laboratory by Yusheng Zhao, a staff member at the Los Alamos Neutron Science Center, and his former postdoctoral associate, Jiang Qian. "The thermal stability, high thermal conductivity, overall toughness, and extreme durability of this material make it a perfect solution for extending the functional life of any tool," says Zhao, who won the Laboratory's 2007 Distinguished Licensing Award for his work transferring this technology. Diamonds are breakable because intrinsic atomic-level misalignments in the stones can initiate cracks that, under stress, become fractures. In the new product, individual diamond crystals are caught in a silicon carbide matrix composed of ultra-tiny crystals (nanocrystals) thousands of times smaller than the diamonds they surround. The matrix stops cracks from growing beyond single diamond crystals, thereby preserving the integrity of the overall composite. Says Zhao, "We developed a two-step synthesis process to ensure that silicon carbide completely surrounds each diamond and that the silicon carbide crystals remain extremely small to exploit the high fracture toughness of nanocrystalline materials." U.S. Synthetic will use the new material for mining and oil- and gas-exploration tools as well as for industrial bearings, wire-drawing dies, heat-sink devices for electronic devices, and other industry applications. Out on a WHIMIn the universe, galaxies are only the tip of the iceberg. Or the tip of the mountain. Brian O'Shea of the Laboratory's Theoretical Division says, "They're like the Pacific islands, which are just the tips of underwater mountains. Galaxies are only the tips of what's really there in the universe." 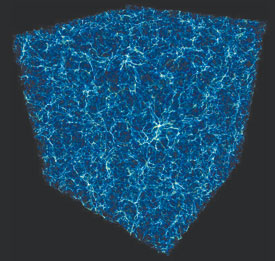
A portion of a supercomputer simulation of the universe, showing the filamentary intergalactic medium.
Normal matter, made of baryons (mostly neutrons and protons), accounts for only 4 to 5 percent of the universe. (The rest of the universe is dark energy and nonbaryonic dark matter, which neither emits nor absorbs light.) Only a small fraction of the baryonic matter is in the galaxies; the rest is in the intergalactic medium, a web of gas, varied in temperature, that stretches between the galaxies. Much of the intergalactic medium has never been detected, so exactly how much baryonic matter exists where is elusive information. To find out, O'Shea and a team of university collaborators (led by the University of Colorado at Boulder) conducted the first supercomputer simulation that included both dark matter and baryonic gas in a model of the universe. The simulation incorporated virtually all of the known physical conditions of the universe and modeled a region more than 1.5 billion light-years across (one light-year equals about six trillion miles of space). The results, reported last December in the Astrophysical Journal, suggested that about 40 percent of the intergalactic medium is in a heretofore-invisible portion called the warm-hot intergalactic medium—the WHIM. Further, the team predicted that about half of the WHIM (20 percent of the intergalactic medium) may eventually be seen, by virtue of its temperature and the wavelength of the radiation it emits. And now someone has actually seen it. While analyzing images from the Hubble Space Telescope and the Far Ultraviolet Spectroscopic Explorer, University of Boulder astronomers Mike Shull and Charles Danforth detected bits of the WHIM dimly superimposed on the backlight of distant quasars. Apparently, the WHIM is able to absorb some of the quasar light, leaving a faint, detectable shadow of itself. Shull and Danforth estimated they were looking at about 25 percent of the intergalactic medium. Is that confirmation of the model? "They said 25 percent; we predicted 20 percent," says O'Shea. "Close enough." —Eileen Patterson
|

Key words-Trichoderma reesei, fungus, biofuel, bees, bomb detection, Proboscis Extension Reflex Platform, WHIM, intergalactic medium, diamond composite, silicon carbide, cutting tools
|