

|

|

Volume 5: No.
2, April 2008
ORIGINAL RESEARCH
Start-Up of the Colorectal Cancer Screening Demonstration Program
Amy DeGroff, MPH, Debra Holden, PhD, Sonya Goode Green, MPH, Jennifer Boehm, MPH, Laura
C. Seeff, MD, Florence Tangka, PhD
Suggested citation for this article: DeGroff A, Holden D, Green SG, Boehm J, Seeff LC, Tangka
F. Start-up of the Colorectal Cancer Screening Demonstration Project. Prev Chronic Dis 2008;5(2).
http://www.cdc.gov/pcd/issues/2008/
apr/07_0204.htm. Accessed [date].
PEER REVIEWED
Abstract
Introduction
In 2005, the Centers for Disease Control and Prevention funded five sites to implement the Colorectal Cancer Screening Demonstration Program (CRCSDP). An evaluation is being conducted
that includes a multiple case study. Case study results for the start-up period, the time between initial funding and screening initiation, provide details about the program models and start-up process and reveal important lessons learned.
Methods
The multiple case study includes all five CRCSDP sites, each representing a unique case. Data
were collected from August 2005 through September 2006 from documents, observations, and more than 70 interviews with program staff and stakeholders.
Results
Sites differed by geographic service area, screening modality selected, and service delivery structure. Program models were influenced by two factors: preexisting infrastructure and the need to adapt programs to fit local service delivery structures. Several sites modeled program components after their National Breast and Cervical Cancer Early Detection Program. Medical advisory boards convened by all sites provided clinical support for developing program policies and quality assurance
plans. Partnerships with comprehensive cancer control programs facilitated access to financial and in-kind resources.
Conclusion
The program models developed by the CRCSDP sites offer a range of prototypes.
Case study results suggest benefits in employing a multidisciplinary staff team,
assembling a medical advisory board, collaborating with local partners, using
preexisting resources, designing programs that are easily incorporated into existing service delivery systems, and planning for adequate start-up time.
Back to top
Introduction
Colorectal cancer is the second leading cause of cancer-related death in the United States (1). Although strong scientific evidence suggests that regular colorectal cancer screening is effective in helping to reduce incidence and mortality from this disease (2), less is known about how to effectively implement colorectal cancer screening in a population-based setting. In this context, the Centers for Disease Control and Prevention (CDC) funded five sites in August 2005 to implement the
Colorectal Cancer Screening Demonstration Program (CRCSDP) for a 3-year period and planned an evaluation to assess its feasibility. The five grantee organizations
are the Maryland Department of Health and Mental Hygiene, the Missouri Department of Health and Senior Services, the Nebraska Department of Health and Human Services, Stony Brook University Medical Center, and Public Health – Seattle & King County.
Before funding the CRCSDP, CDC used Framework for Program Evaluation in Public Health (3) to develop an evaluation plan with three purposes: 1) understanding program implementation (processes); 2) measuring program effects (outcomes) at the individual client level, and 3) assessing program efficiencies (costs). CDC adopted a goal-based (4), utilization-focused (5) evaluation approach and developed evaluation questions, consistent with the purposes above, for each of eight CRCSDP
program goals, which were defined on the basis of the program components. CDC selected three methods to evaluate the CRCSDP: 1) a multiple case study, 2) the collection and analysis of clients’ screening and diagnostic services data, and 3) a costs and cost-effectiveness analysis. CDC is collecting and analyzing data for two distinct periods: 1) program start-up (i.e., the time between initial funding and the initiation of screening services) and 2) screening implementation. This
report summarizes case study results for the start-up period, describes the five unique program models and the start-up process, and identifies important lessons learned.
Back to top
Methods
The study team conducted a multiple case study to better understand program implementation processes and to describe the experience and context of each CRCSDP program. A multiple case study approach was used in part because it would allow comparisons
between the five sites. All five CRCSDP programs were included in the multiple case study (6,7), each representing a unique case.
Table 1 presents the eight CRCSDP program goals and offers examples of evaluation questions addressed by the case
study.
Data collection
The study team collected data from documents, interviews, and observations from August 2005 through September 2006. Key documents were summarized
by using a structured guide, and other documents were retained in their entirety. Documents included funding proposals to CDC for the first 2 years of the CRCSDP
program, program policies, patient flowcharts, and minutes from an all-site
conference call. In February and March 2006, the team conducted a telephone
interview, using a semistructured interview guide, with the program director for
each site; three in-person interviews were also conducted with CDC program consultants who provided technical assistance and other support to the sites.
The team made 2-day visits to each program site during summer 2006 to record observations and conduct interviews with staff and stakeholders. Ten unique, semistructured interview guides were developed for the following positions: bureau chief, program director, program coordinator, quality assurance coordinator, outreach coordinator, epidemiologist, medical advisory board (MAB) member, provider site coordinator, endoscopist, and Comprehensive Cancer Control (CCC) coordinator or other
partner. The team identified these roles on the basis of typical staffing patterns among the sites and program policies imposed by CDC (e.g., programs must convene an
MAB). Interview questions were developed on the basis of the role of the interviewee, the evaluation questions, and information gathered during the earlier interviews with program directors and CDC program consultants. The team used purposeful sampling to select interviewees who were likely to provide the most
in-depth information (5); relevant stakeholders were identified with assistance from program staff. A team of two evaluators conducted most interviews, which were audiotaped and lasted approximately 60 minutes. The team conducted a total of 67 interviews
(30 staff and 37 stakeholders). On the basis of informal observations conducted at all sites, descriptive field notes were developed.
Analysis
Data analysis involved an iterative approach whereby team members regularly met to discuss impressions, review field notes, identify themes, and consider areas of emphasis for subsequent interviews (8). The team transcribed all interviews and entered them along with documents and document summaries into Atlas.ti (Atlas.ti Scientific Software Development GmbH, Berlin, Germany), a software program for qualitative data analysis. Categories and themes were developed both inductively from the
data (e.g., challenges in recruiting endoscopists) and deductively from the evaluation questions (e.g., description of partnership activities). The team developed and refined a codebook with detailed code definitions. A single evaluator was assigned to code all interviews for one program site. The team coded 65 of the 67 on-site interviews, excluding two interviews because the interviewees were unfamiliar with details of their sites’ CRCSDP program.
Because of resource limitations, the documents were not coded, nor were
the five telephone interviews with program directors or the three interviews with program consultants, but these materials were used in the analysis.The team met twice weekly during the coding process to discuss issues and review the memos of each team member. A second team member coded half of all interviews for each site; the two coders discussed discrepancies to make final coding decisions. The constant comparative method (9) was used to compare categories of
data at different levels. Inferences from the coded data were made using content analysis (10). The team developed typologies (e.g., classifying service delivery models) and tables as an additional way to understand the organizational arrangements and service delivery processes (11). Finally, within-case analysis (6) was conducted for each of the five programs, and case-specific reports were developed.
Credibility
Each member of the evaluation team engaged in all aspects of data collection
and analysis, an approach that contributed to a thorough and holistic
understanding of each case. Both methodologic and data-source triangulation were used to verify findings; using more than one source of evidence is known to strengthen findings (11-14). The team maintained a detailed audit trail documenting the research methods and process to ensure transparency (13). Finally, the process of member checking was used for
the in-case analysis (12,15); this process engages research participants in a review of tentative findings to verify their accuracy.
Back to top
Results
We present results for two distinct areas. The first, program models, summarizes characteristics of each CRCSDP program model. The second, program processes, presents data related to key start-up processes.
Program models
The five sites differed in geographic service area: two served a city
(Baltimore, Maryland, and St. Louis, Missouri); two served counties (Suffolk County, New York; and King, Clallam, and Jefferson counties, Washington); and one served a state (Nebraska).
Missouri, Nebraska, and Washington planned to use the guaiac-based fecal occult blood test (FOBT) as the primary screening test, with colonoscopy
being used for diagnosis and screening of high-risk people (Table 2).
Maryland and New York planned to use colonoscopy as their primary screening
test. On the basis of CDC guidelines related to the priority population for the program, we found consistency
between
the populations served by the five programs.
The organizational relationships for the programs’ service delivery systems
varied (Figures 1–5). Nebraska and New York planned to deliver screening services
themselves. Maryland, Missouri, and Washington, however, planned to provide program oversight
and contract with other agencies to deliver screening services. Missouri, Nebraska, and New York planned centralized service delivery systems,
but Maryland and Washington planned decentralized systems.
The Missouri Department of Health and Senior Services planned to contract with a provider in St. Louis to assess client eligibility for screening, deliver FOBT services, track and follow up on clients, and provide colonoscopies (Figure 1). The Nebraska Department of Health and Human Services planned to assess client eligibility for screening and deliver FOBT services,
but to contract with outside providers for tracking and follow-up, laboratory, and colonoscopy services (Figure 2). Stony Brook
University Medical Center in New York represents an enclosed system in which departments within the medical center planned to conduct all aspects of service delivery (Figure 3).
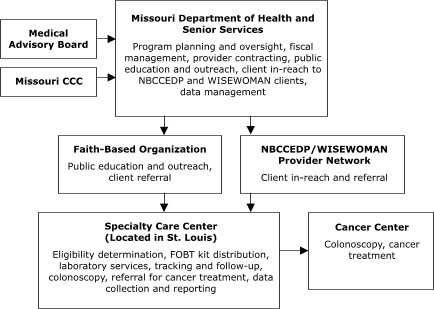
Figure 1. Centralized provider system for the Colorectal Cancer Screening Demonstration Program, Missouri. Both the specialty care center and cancer center provide endoscopic services. CCC indicates Comprehensive Cancer Control; NBCCEDP, National Breast and Cervical Cancer Early Detection Program;
WISEWOMAN, Well-Integrated Screening and Evaluation for Women Across the
Nation; FOBT, fecal occult blood test.
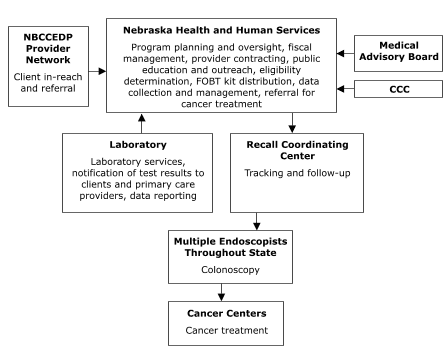
Figure 2. Centralized provider system for the Colorectal Cancer Screening Demonstration Program, Nebraska. NBCCEDP indicates National Breast and Cervical Cancer Early Detection Program; FOBT, fecal occult blood test; CCC, Comprehensive Cancer Control.
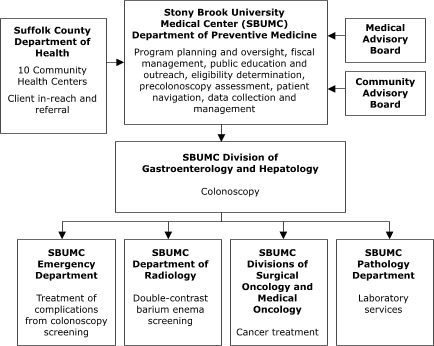
Figure 3. Centralized Provider System for the Colorectal Screening Demonstration Program, New York.
Of the sites with decentralized models of service delivery, the Maryland Department of Health and Mental Hygiene (Figure 4) planned to contract with five hospitals, each of which would provide all elements of screening service. In Washington, Public Health – Seattle & King County planned to contract with 10 primary care centers to assess screening eligibility, deliver FOBT services, ensure tracking and follow-up, and provide laboratory services (Figure 5).
The plan also called for contracting with 1) another agency to provide patient navigation services to people referred for colonoscopy and
2) several endoscopists to conduct colonoscopy. In general, staff members in Maryland and Washington valued the decentralized model for its community-based orientation but perceived the model as more difficult to establish because of the need to support multiple sites in integrating and adapting the program into their existing service delivery
systems.
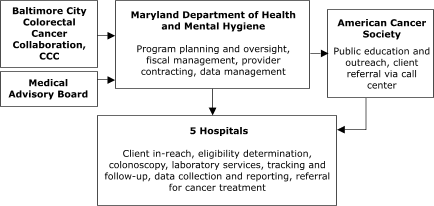
Figure 4. Decentralized provider system for the Colorectal Cancer Screening Demonstration Program, Maryland. CCC indicates Comprehensive Cancer Control.
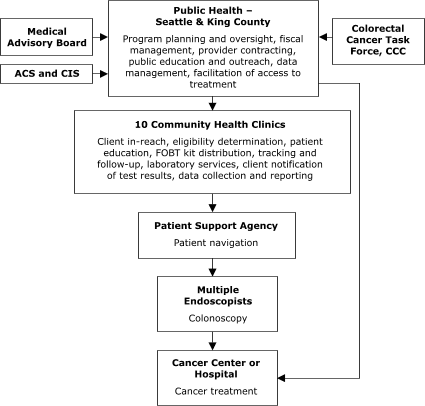
Figure 5. Decentralized provider system for the Colorectal Cancer Screening Demonstration Program, Washington. ACS indicates American Cancer Society; CIS, Cancer Information System; CCC, Comprehensive Cancer Control; FOBT, fecal occult blood test. The Colorectal Cancer Task Force is a subcommittee of the statewide CCC that was established to address colorectal cancer issues.
Two key factors influenced the program design of all five programs. First, several sites developed the new CRCSDP, or components of it, from existing programs such as the National Breast and Cervical Cancer Early Detection Program (NBCCEDP). For instance, sites planned to use NBCCEDP provider networks to support client in-reach or to distribute FOBT kits and were in the process of integrating other program components with existing NBCCEDP components. One staff member noted, “The easy part
for us was having a screening and tracking system in place already that we were comfortable with [NBCCEDP]. . . . We were able to use similarities in our existing system and customize those for CRCSDP.” The second factor influencing the CRCSDP program models was the need for sites to tailor service delivery systems in ways that facilitated their integration into existing clinical structures. Participants said such integration was necessary to minimize the burden and disruption for participating
clinical sites. For decentralized models, the need to “fit” the provider context resulted in unique patient flow patterns at multiple provider settings.
Start-up processes
The start-up process lasted 9 to 11 months and involved assembling a staff team, developing program models, convening a MAB to assist in developing policies and procedures, building partnerships, planning for client recruitment, developing a data management system, and identifying resources for the treatment of complications.
Staffing
Each program recruited a team of two or three people, usually from existing
positions, to assist in developing the new program. Teams typically included a program director, a program coordinator working on day-to-day activities, and a data management specialist. Programs that were able to easily access staff with clinical expertise within the grantee organization noted the importance of being able to do so. Nearly all CRCSDP program directors were also managing their state or
region’s NBCCEDP, and some were managing a Well–Integrated Screening and
Evaluation for Women Across the Nation (WISEWOMAN) program, another CDC-funded screening program (16). Program directors had extensive program and management experience and preexisting partner relationships with cancer prevention and control leaders in their state. The team approach helped ensure that enough people with varied expertise were available to attend to the many start-up responsibilities.
Medical advisory board
An MAB was convened by each program and provided essential clinical guidance during the start-up period, especially for CRCSDP sites lacking staff with extensive medical expertise in colorectal cancer. MAB composition varied by site but largely reflected clinical disciplines relevant to colorectal cancer and screening, including primary care specialists, gastroenterologists, and radiologists
(Table 3). One respondent suggested that the prescription for a well-rounded MAB includes
“basically anybody
involved in any step of the way from screening to diagnosis to treatment, a continuum of care, with a heavy emphasis on GI [gastrointestinal specialists].” The MABs served as a functional work group, providing direction on policy development, program eligibility criteria, patient flow, data collection, and quality assurance. MABs participated informally, meeting as a group
infrequently but otherwise being accessible to program staff by telephone and e-mail.
Partnerships
Partnerships provided critical resources, both financial and in-kind, and played an active role during program start-up. Key partners included state or regional CCC groups, the American Cancer Society (ACS), community-based organizations, and universities.
Several partners provided in-kind staff support, and CCC groups contributed financial resources for a public education campaign in one site and database development in two others. CCC groups were also valuable in
negotiating
relationships with MAB members, endoscopists, and representatives of clinical provider sites. An ACS call center planned to recruit CRCSDP
clients for one program, and a local university planned to assist with client
recruitment and evaluation in another.
Client recruitment
CRCSDP sites planned public education, outreach, and in-reach strategies to recruit clients for screening (Table 4). Several sites adopted CDC’s Screen for Life: National Colorectal Cancer Action Campaign or ACS public education materials. Staff emphasized the use of culturally sensitive public education materials. Although public education efforts were intended to raise awareness about, and create demand for, the new CRCSDP, interviewees expressed apprehension about creating too great
a demand for screening services early in program implementation. Staff planned to begin with a slow process of recruitment so they could test their systems. Ten Suffolk County community health centers collaborated with the New York program during the start-up period to
develop a plan for referring clients for screening. Other CRCSDP sites focused on developing in-reach efforts to recruit clients from existing screening programs such as the NBCCEDP. However, interviewees expressed concerns about
recruiting men for the CRCSDP through NBCCEDP, observing that men generally are less likely to access preventive health care services. One stakeholder noted the following:
“All of the people from the men’s health sector say that the only thing men say is that
‘my wife made me do it’ [get screened]. All of the doctors say that, too, that men say their wives made them come in. But we don’t want to put all of that burden on women. Women are used to getting screenings and doing preventive care; it’s not part of the culture for men.”
Data management systems
During program start-up, CDC, in collaboration with the five CRCSDP sites,
developed a set of colorectal clinical data elements to collect patient-level
demographic, screening, and diagnostic data on program clients. Whereas one CRCSDP site developed a new data system, others augmented existing systems (e.g., the NBCCEDP data system) to integrate the
data elements. With support from MABs and provider sites, each program also developed data collection forms (e.g., patient enrollment, health
history, FOBT screening). Although staff suggested that the development of data systems and forms was not particularly difficult, they observed that it was an especially time-consuming component of the start-up period.
Treatment resources
Staff identified challenges in securing resources for cancer treatment. Because CDC funds cannot be used for treatment (17), programs depended on soliciting in-kind support or charity care from a provider system viewed by staff as already overburdened.
Back to top
Discussion
The program models and start-up process of the CRCSDP offer valuable insight to those with an interest in developing colorectal cancer screening programs. Several key factors emerged from the evaluation of the start-up experience of the five sites studied here. These factors include use of a multidisciplinary team, involvement of an MAB, relationships with partners, the use of preexisting resources, a program model that fits existing service delivery systems, and adequate planning
time.
In these five programs, two to three staff with expertise in program management and administration (e.g., collaboration, contracting, policy development), program coordination (e.g., day-to-day management, training, support), and data management (e.g., data systems, data form development) provided an adequate team for program start-up. Clinical expertise and comfort discussing clinical issues with MAB members and service providers were important skills for the management team.
Access to clinicians with expertise in colorectal cancer was essential to start-up. A well-rounded MAB that included professionals in disciplines related to the screening process (e.g., endoscopists, pathologists, radiologists, surgical oncologists, social workers, community-based practitioners) was beneficial.
CDC and other organizations recognize that public health problems demand collaborative efforts rather than
“going it alone” (18,19). Active and extensive partnerships were fundamental in helping the programs plan to recruit clients, increase public awareness about the need for screening, and facilitate relationships with MAB members and screening sites.
The five CRCSDP sites leveraged existing resources to build a new colorectal cancer screening program. Partner agencies (e.g., CCC, ACS), other screening programs (e.g., NBCCEDP), and internal agency departments (e.g., health communications, epidemiology) helped reduce costs and support program development. The length of time needed to develop data systems and data collection forms suggests new programs may benefit from using existing data forms and data collection sets.
These five programs used program models that would most easily integrate into existing service delivery systems. For the decentralized models, integration involved allowing for varied implementation approaches within multiple service delivery sites for the same program (e.g., five different clinical sites providing colonoscopy screening). Reliance on in-reach to NBCCEDP clients and overall concerns about effectively recruiting men suggest programs may need to consider program models that
include unique recruitment efforts for men.
Although CDC had anticipated a 6-month start-up period, these programs needed
9 to 11 months to hire staff, convene an MAB,
develop policies, build partnerships, organize a service delivery system, plan
for client recruitment, secure treatment resources, and develop data management
systems. One staff member advised, “The devils are in the details — all the little
things that you have to think through that we didn’t even think of — things we
thought we knew but we didn’t.”
The CRCSDP evaluation team will continue to work with the five sites as they provide colorectal cancer screening to low-income, underserved communities. The case study, in particular, contributes to important process evaluation efforts that improve our understanding of the CRCSDP’s program operations, implementation, and service delivery (20). Recognizing that the potential for evaluation to effect change is dependent on its use (21), evaluators encourage others with an interest in
colorectal cancer screening to consider the results presented here.
Back to top
Acknowledgments
We thank participating CRCSDP staff and stakeholders and CDC program consultants for their generous contribution of time and cooperation in the case study evaluation. We also thank CDC’s CRCSDP Project Team for ongoing support and their review of the manuscript.
Back to top
Author Information
Corresponding Author: Amy DeGroff, MPH, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, 4770 Buford Hwy NE, MS K-57, Atlanta, GA 30341. Telephone: 770-488-2415. E-mail: asd1@cdc.gov.
Author Affiliations: Debra Holden, Sonya Goode Green, Research Triangle Institute, Research Triangle Park, North Carolina; Jennifer Boehm, Laura Seeff, Florence Tangka, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia.
Back to top
References
- U.S. Cancer Statistics Working Group. United States cancer statistics: 2003
incidence and mortality. Atlanta (GA): U.S. Department of Health and Human Services,
National Cancer Institute, Centers for Disease Control and Prevention; 2007.
- Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al.
The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343(22):1603-7.
-
Framework for program evaluation in public health. MMWR Recomm Rep
1999;48(RR-11):1-40.
- Stufflebeam DL. Evaluation models. New Dir Eval 2001;(89):1-106.
- Patton MQ. Qualitative research & evaluation methods. 3rd ed. Thousand Oaks (CA): SAGE Publications; 2002.
- Stake RE. The art of case study research. Thousand Oaks (CA): SAGE Publications; 1995.
- Stake RE. Multiple case study analysis. New York (NY): Guilford Press; 2006.
- Bogdan RC, Biklen SK. Qualitative research for education: an introduction to theories and methods. Boston (MA): Allyn and Bacon; 2007.
- Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago (IL): Aldine; 1967.
- Krippendorf K. Content analysis: an introduction to its methodology. Beverly Hills (CA): SAGE Publications; 1980.
- Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. Thousand Oaks (CA): SAGE Publications; 1994.
- Creswell JW, Miller DL. Determining validity in qualitative inquiry. Theory into Practice 2000;39(3):124-30.
- Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park (CA): SAGE Publications; 1985.
- Mathison S. Why triangulate? Educational Researcher 1988;17(2):13-7.
- Merriam SB. Qualitative research and case study applications in education. San Francisco (CA): Jossey-Bass; 1998.
- WISEWOMAN — Well–Integrated Screening and Evaluation for Women Across the
Nation. Atlanta (GA): Centers for Disease Control and Prevention. http://www.cdc.gov/wisewoman.
Accessed September 10, 2007.
- Colorectal Cancer Screening Demonstration Program. Fed Regist 2005;70(99):29747-59.
- CDC health protection goals fact sheet: goals for the 21st century.
Atlanta (GA): Centers for Disease Control and Prevention. http://www.cdc.gov/about/goals/factSheet.htm.
Accessed September 10, 2007.
- Institute of Medicine. The future of the public’s health in the 21st
century. Washington (DC): National Academies Press; 2003.
- Rossi PH, Freeman HE, Lipsey MW. Evaluation: a systematic approach. 6th ed. Thousand Oaks (CA): SAGE Publications; 1999.
- Weiss CH. Evaluation. 2nd ed. Upper Saddle River (NJ): Prentice Hall; 1998.
Back to top
|
|
