In this Issue:
1. NIDCR News
2. NIH News
3. Funding Opportunities
4. Science Advances
NIDCR NEWS
Dr. Harold Varmus to Deliver 2008 David E. Barmes Global Health Lecture
 Dr. Harold Varmus, president of Memorial Sloan-Kettering Cancer Center in New York, will deliver the 2008 David E. Barmes Global Health Lecture on Tuesday, December 16 at 11:30 a.m. on the NIH campus. Former director of the National Institutes of Health and co-recipient of a Nobel Prize for studies of the genetic basis of cancer, Dr. Varmus has served as the president and chief executive officer of Memorial Sloan-Kettering since January 2000.
Dr. Harold Varmus, president of Memorial Sloan-Kettering Cancer Center in New York, will deliver the 2008 David E. Barmes Global Health Lecture on Tuesday, December 16 at 11:30 a.m. on the NIH campus. Former director of the National Institutes of Health and co-recipient of a Nobel Prize for studies of the genetic basis of cancer, Dr. Varmus has served as the president and chief executive officer of Memorial Sloan-Kettering since January 2000.
The annual lecture series honors the late David E. Barmes, a longstanding World Health Organization employee, special expert for international health at NIDCR, and ardent spokesman for global health. The lecture, which is jointly sponsored by the NIDCR and the Fogarty International Center, will be videocast.
NIDCR Now Accepting Applications for 2009 Summer Dental Student Award Program
To expose future dentists to careers in research, NIDCR offers an outstanding summer training opportunity for dental students. The NIDCR Summer Dental Student Award is designed to give talented dental students hands-on research experience and exposure to the latest advances in oral health research. Selected candidates are assigned to mentors who conduct research in the students’ areas of interest. Participation in the program may result in presentation of research findings at a scientific meeting or co-authorship of scientific publications.
NIDCR provides a competitive stipend for all summer researchers. Acceptance of this award requires a minimum eight-week commitment. The nominating dental school must agree to support air or ground transportation for their students.
Online applications for the Summer Dental Student Award will be accepted from November 15, 2008 – January 15, 2009. See additional information about the Summer Dental Student Award or contact Dr. Deborah Philp, Program Director, dphilp@dir.nidcr.nih.gov, (301) 594-8449
Dr. Lawrence Tabak Appointed Acting Principal Deputy Director of NIH
 With the departure of Dr. Elias Zerhouni in October, his principal deputy, Dr. Raynard Kington, was appointed Acting Director of NIH. On November 12, Dr. Kington appointed Dr. Lawrence Tabak as Acting Principal Deputy Director of NIH. While serving in this temporary role, Dr. Tabak will continue to direct the NIDCR.
With the departure of Dr. Elias Zerhouni in October, his principal deputy, Dr. Raynard Kington, was appointed Acting Director of NIH. On November 12, Dr. Kington appointed Dr. Lawrence Tabak as Acting Principal Deputy Director of NIH. While serving in this temporary role, Dr. Tabak will continue to direct the NIDCR.
NIDCR Director on the Future of Dentistry
NIDCR Director Lawrence Tabak recently spoke to the Canadian Dental Association about how research will influence the future of dentistry. Listen to his interview or read the transcript.
Job Openings
The following positions are available at the NIDCR:
Health Scientist Administrator, Division of Extramural Research, Translational Genomics Research Branch
Health Scientist Administrator, Division of Extramural Activities, Scientific Review Branch
Health Scientist Administrator, Division of Extramural Research, Integrative Biology and Infectious Diseases Branch, Salivary Biology and Immunology Program
Clinical Director, Division of Intramural Research
Chief, Dental Consult Services (Staff Clinician)
Oral-Maxillofacial Surgeon, Division of Intramural Research
See additional details about how to apply.
NIH NEWS
NIH Announces Initial Implementation Timeline for Enhancing Peer Review 
On September 30, NIH announced that it will begin implementing changes to enhance its peer review system, after an extensive year-long review. External and internal working groups--co-chaired by NIDCR Director Lawrence Tabak--deliberated on challenges and recommendations regarding enhancements to the review system. The resulting set of recommendations led to changes in four core priority areas:
- Continue to Engage the Best Reviewers
- Improve the Quality and Transparency of Review
- Ensure Balanced and Fair Reviews across Scientific Fields and Career Stages, and Reduce Administrative Burden
- Continuous Review of Peer Review
See additional information about the peer review changes.
Also see the peer review website.
New NIH Policy to Fund Meritorious Science Earlier
NIH has announced a new policy to ensure earlier funding of high quality applications and improve efficiency in the NIH peer review system. Beginning January 25, 2009, NIH will decrease the number of times an amended grant application may be resubmitted from two to one. The new policy applies both to new applications and competing renewal applications. Failure to receive funding after two submissions (the original and the single amendment) will mean that the applicant should substantially redesign the project rather than simply change the application in response to previous reviews.
See the news release about the new resubmission policy and the related Notices:
http://grants.nih.gov/grants/guide/notice-files/NOT-OD-09-003.html
http://grants.nih.gov/grants/guide/notice-files/NOT-OD-09-016.html
New NIH Policy Establishes Goals to Support Scientists Early in Their Careers
NIH has issued a policy establishing goals to encourage funding for scientists new to NIH and those who are at an early stage in their careers. The aim is to allow new investigators to achieve success rates comparable to those of established scientists submitting grant applications. NIH hopes to support 1,650 or more new investigators across all Institutes and Centers in FY 2009.
As a first step, NIH has created a new "Early Stage Investigator" category to accelerate the early transition of new scientists to research independence. Early stage investigators are defined as new or first-time investigators who are within 10 years of completing their last research degree or are within 10 years of completing their medical residency (or equivalent). See the related Notice.
Early stage investigators seeking NIH funding for the first time are encouraged to apply for research project grants (R01s), rather than small grants (R03s) or exploratory/developmental grants (R21s) that are limited in scope and period of support. See the Notice about revised new and early stage investigator policies.
NIH Seeks Proposals for 2009 Director's Pioneer and New Innovator Awards
 NIH welcomes proposals for 2009 NIH Director's Pioneer Awards and New Innovator Awards. Both programs are part of the NIH Roadmap for Medical Research and support exceptionally creative scientists who take highly innovative, potentially high-impact approaches to major challenges in biomedical or behavioral research.
NIH welcomes proposals for 2009 NIH Director's Pioneer Awards and New Innovator Awards. Both programs are part of the NIH Roadmap for Medical Research and support exceptionally creative scientists who take highly innovative, potentially high-impact approaches to major challenges in biomedical or behavioral research.
Pioneer Awards provide up to $2.5 million in direct costs over 5 years and are open to scientists at any career stage. New Innovator Awards provide up to $1.5 million in direct costs over the same period and are for early career investigators who have not received a research project grant (R01) or similar NIH grant.
NIH expects to make 5 to 10 Pioneer Awards and up to 24 New Innovator Awards in September 2009. To continue its strong record of diversity in these programs, NIH especially encourages wome n and members of groups that are underrepresented in NIH research to apply.
n and members of groups that are underrepresented in NIH research to apply.
Starting this year, both the Pioneer Award and the New Innovator Award competitions will begin with a pre-application phase. The Pioneer Award competition will have a proposal submission period from November 17 to December 17, 2008.
See further instructions about the pre-application phase.
Also see the Request for Applications and additional information about the Pioneer Award.
The New Innovator Award competition begins with a proposal submission period from December 15, 2008 to January 15, 2009. See details about the new application process.
Also see the Request for Applications.
Notice of Clarification of Eligibility Criteria
Additional information about the New Innovator Award
New Transformative R01 Program
NIH's new Transformative R01 Program (T-R01s) will allow highly  creative, "out-of-the-box" projects to be supported. The T-R01 represents a High Risk/High Reward Demonstration Project in which novel approaches to peer review and program management are to be piloted.
creative, "out-of-the-box" projects to be supported. The T-R01 represents a High Risk/High Reward Demonstration Project in which novel approaches to peer review and program management are to be piloted.
The application submission period is from December 29, 2008 to January 29, 2009.
See more more information about the new Transformative R01 Program.
Human Microbiome Project Awards
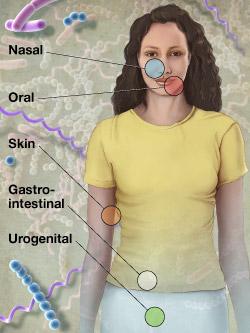 NIH has announced the first awards for its Human Microbiome Project, which will lay a foundation for efforts to explore how complex communities of microbes interact with the human body to influence health and disease. The funding, estimated to be up to approximately $21.2 million for 2- and 3-year projects, will support the development of innovative technologies and computational tools, coordination of data analysis, and an examination of some of the ethical, legal and social implications of human microbiome research. See the news release about the Human Microbiome Project Awards.
NIH has announced the first awards for its Human Microbiome Project, which will lay a foundation for efforts to explore how complex communities of microbes interact with the human body to influence health and disease. The funding, estimated to be up to approximately $21.2 million for 2- and 3-year projects, will support the development of innovative technologies and computational tools, coordination of data analysis, and an examination of some of the ethical, legal and social implications of human microbiome research. See the news release about the Human Microbiome Project Awards.
The Human Microbiome Project is part of the NIH Roadmap for Medical Research and is managed by the National Institute of Allergy and Infectious Diseases, NIDCR, the National Institute of Diabetes, Digestive and Kidney Diseases and the National Human Genome Research Institute. The Roadmap is a series of initiatives designed to pursue major opportunities and gaps in biomedical research that no single NIH institute could tackle alone. See additional information about the NIH Roadmap.
NIH Announces Funding for New Epigenomics Initiative
NIH has announced funding for the new NIH Roadmap Epigenomics Program. Epigenetic processes control normal growth and development, and epigenomics is a study of epigenetic processes at a genome-wide scale. The NIH will invest more than $190 million over the next five years to accelerate this emerging field of biomedical research. The first grants will total approximately $18 million in 2008.
The overall hypothesis of the NIH Roadmap Epigenomics Program is that the origins of health and susceptibility to disease, are, in part, the result of epigenetic regulation of the genetic blueprint. Researchers believe that understanding how and when epigenetic processes control genes during different stages of development and throughout life will lead to more effective ways to prevent and treat disease. See more information about the epigenomics initiative.
NIH's Genes Environment and Health Initiative Adds Six Studies
The NIH Genes, Environment and Health Initiative (GEI) has awarded grants estimated to be up to $5.5 million over two years for six studies aimed at finding genetic factors that influence the risks for stroke, glaucoma, high blood pressure, prostate cancer and other common disorders. The grantees will use a genome-wide association study to rapidly scan markers across the complete sets of DNA, or genomes, of large groups of people to find genetic variants associated with a particular disease, condition or trait.
GEI is a collaboration between genetic researchers and environmental scientists. Six GEI-supported genome-wide association studies, overseen by the National Human Genome Research Institute, are already under way. Two additional GEI studies, supported and managed by the NIDCR, have also begun. See additional information about the Genes Environment and Health Initiative.
NIH to Hold Health Disparities Summit
 On December 16-18, an NIH summit on "The Science of Eliminating Health Disparities" will take place at the Gaylord National Resort & Convention Center in National Harbor, MD. The event will showcase NIH's collective contribution to developing new knowledge in the science of eliminating health disparities. The summit will feature: plenary sessions highlighting the intersections of science, practice, and policy in health disparity research; interactive breakout sessions organized into five tracks to examine health disparities from multiple disciplines; an awards banquet to recognize individuals who have demonstrated extraordinary contributions towards the elimination of health disparities; and a town hall meeting to gather input from audience members. Registration for the summit is free and available online.
On December 16-18, an NIH summit on "The Science of Eliminating Health Disparities" will take place at the Gaylord National Resort & Convention Center in National Harbor, MD. The event will showcase NIH's collective contribution to developing new knowledge in the science of eliminating health disparities. The summit will feature: plenary sessions highlighting the intersections of science, practice, and policy in health disparity research; interactive breakout sessions organized into five tracks to examine health disparities from multiple disciplines; an awards banquet to recognize individuals who have demonstrated extraordinary contributions towards the elimination of health disparities; and a town hall meeting to gather input from audience members. Registration for the summit is free and available online.
NIH Grantees Win 2008 Nobel Prizes
The 2008 Nobel Prize in chemistry is shared by two NIH grantees, Martin Chalfie, Ph.D. of Columbia University and Roger Y. Tsien, Ph.D., of the University of California at San Diego. The two researchers share the award with a former NIH grantee, Osamu Shimomura, Ph.D., of the Marine Biology Laboratory in Woods Hole, MA. The three researchers are honored for discovering a fluorescent protein, GFP, in a colorful jellyfish and developing it into a key tool for observing previously invisible processes, such as the development of nerve cells in the brain or how cancer cells spread. By using DNA technology, researchers can now connect GFP to other interesting, but otherwise invisible proteins. The glowing marker allows them to watch the movements, positions, and interactions of the targeted proteins. See additional information about the Nobel grantees.
NIH Launches New Web Site for Parents on Medical Research Studies for Children
 From asthma and cancer treatments to vaccines, research in children saves lives and improves their health and well-being. A new web site from NIH, "Children and Clinical Studies," offers parents and health care providers an insider's guide to children's medical research. The web site combines information about how clinical studies in youth are conducted with award-winning video of children, parents, and healthcare providers discussing the rewards and challenges of participating in research. See the new web site on Medical Research Studies for Children.
From asthma and cancer treatments to vaccines, research in children saves lives and improves their health and well-being. A new web site from NIH, "Children and Clinical Studies," offers parents and health care providers an insider's guide to children's medical research. The web site combines information about how clinical studies in youth are conducted with award-winning video of children, parents, and healthcare providers discussing the rewards and challenges of participating in research. See the new web site on Medical Research Studies for Children.
Dr. Li Steps Down as NIAAA Director
Ting-Kai Li, M.D., director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) since 2002, retired at the end of October. Kenneth R. Warren, Ph.D., the NIAAA Deputy Director since February 2008, is serving as acting director of the Institute while a search for a new Director is initiated. See additional details about Dr. Li's retirement.
FUNDING OPPORTUNITIES
PROGRAM ANNOUNCEMENTS
NIH Roadmap
REQUESTS FOR APPLICATIONS
Gene Studies
Neurobiology/Pain
NIH Roadmap
New Concept Clearance
Concepts represent early planning stages for initiatives in which NIDCR seeks to support research in an understudied and significant area of science. Council approval does not guarantee that a concept will become a program announcement (PA), request for applications (RFA), or request for proposals (RFP). NIDCR bases this determination on scientific and programmatic priorities balanced with the amount of funds available.
A concept clearance on "Support for NIDCR Salivary Gland Tumor Biorepository" was
presented at the September meeting of the National Advisory Dental and Craniofacial Council.
NOTICES
SCIENCE ADVANCES
Periodontal Disease: Engineering the Future of Care
 Read the latest online interviews with an NIDCR grantee and intramural scientist to get their perspective on research challenges and opportunities in the 21st Century. See Periodontal Disease: Engineering the Future of Care.
Read the latest online interviews with an NIDCR grantee and intramural scientist to get their perspective on research challenges and opportunities in the 21st Century. See Periodontal Disease: Engineering the Future of Care.
Scientists Identify Gene Variant Involved in Isolated Cleft Lip
 About 20 percent of isolated cleft lip, one of the world’s most common birth defects, may be due to a one-letter difference in the DNA sequence of a gene involved in facial development, researchers supported by the NIDCR and several NIH institutes report. The scientists say the discovery, published in the journal Nature Genetics, could lead to DNA tests to help couples better gauge their risk of having a child with an isolated cleft. Read more about this research on isolated cleft lip.
About 20 percent of isolated cleft lip, one of the world’s most common birth defects, may be due to a one-letter difference in the DNA sequence of a gene involved in facial development, researchers supported by the NIDCR and several NIH institutes report. The scientists say the discovery, published in the journal Nature Genetics, could lead to DNA tests to help couples better gauge their risk of having a child with an isolated cleft. Read more about this research on isolated cleft lip.
Science News in Brief
See the following summaries of recent oral health research findings:
NIDCR Scientists Find Pain Receptor Enhancer
Researchers Assemble Panel of Salivary Biomarkers
New Automated Approach Analyzes Protein Structure