A Look Inside the National Cancer Institute Budget Process: Implications for 2007 and BeyondArticle by Dr. John E. Niederhuber
(Reprinted from Cancer Research 2007; 67: [3]. February 1, 2007)
Introduction
Despite what may be one of the most difficult times in the long
and distinguished history of the NIH, the National Cancer Institute's
(NCI) commitment to its mission of advancing cancer research
cannot falter. Federal deficits resulting from a host of unanticipated
fiscal pressures in the years since September 11, 2001 - the wars in
Afghanistan and Iraq, increased investments in national defense,
research directed at bioterrorism, hurricane Katrina, and the threat
of pandemic flu - have collectively placed significant stress on the
resources available for and assigned to support the country's
biomedical research enterprise. In facing these fiscal challenges, the
NCI has a unique opportunity to streamline its approach, increase
clarity on its long-term goals, and set the stage for biomedical
research to flourish in any financial environment.
The biggest challenge, and the foremost driver of uncertainty to the
biomedical research community, is the discretionary portion of the
annual Federal budget appropriation supporting the NIH. The
amounts and allocations of those funds have been the topic of
discussion at scientific meetings, in the editorial pages of peer-reviewed
journals, in the national media, and among patient and
professional organizations as well as researchers and administrators
at academic centers across the country. This attention to stewardship
of the budget has permeated the biomedical research community.
It is for these reasons that it is important for the entire cancer
community to better understand the stresses on the budget and,
more specifically, the process the NCI is using to optimally invest in
science and to sustain the momentum brought about by the
doubling of the NIH budget. The cancer community can only
benefit from an open discussion of the factors that influence the
budget, and the processes and procedures the NCI has instituted to
effectively manage its resources.
Some History: the Decision to Double the NIH
Budget
The passionate, strong voice of the cancer community provided a
major impetus for the significant increase in the nation's investment
in biomedical research. On September 26, 1998, thousands of
cancer patients, survivors, and advocates gathered at the National
Mall in Washington D.C. to advocate for a renewed commitment
to eliminating the burden of cancer and a greater investment
in biomedical research. As a result of this campaign, Congress
generously stepped forward, and over the next 5 years, beginning in
fiscal year (FY) 1999 (using FY 1998 as the base), doubled the NIH
budget. Although the NCI budget did not completely double, it
did grow by 80% from $2.5 billion (B) to $4.6 B over this period.
This laudable increase produced many favorable outcomes.
Individual NCI investigators were able to expand their research as
the average R01 grant increased nearly 30% to an average total
amount of $346,000. There was increased interest on the part of
university investigators in becoming involved in cancer research.
Even more significantly, university leaders recognized the opportunity
to invest in new laboratory buildings, to build new research programs,
and to add new faculty positions. Dr. Elias Zerhouni, Director of the
NIH, has recounted the story that the common standard of recent
success for a university dean among his or her peers was the number
of construction cranes towering above campus. You were at the top
as a four-crane dean, or just getting by as a one-crane dean.
This anecdote provides a strong dose of reality. Investment in the
NIH achieved a much desired increase in biomedical research, with
more faculty now focused on solving the mechanisms of disease
processes and more laboratories to train the next generation of
investigators. The American Association of Medical Colleges
(AAMC) estimates that between 1998 and 2002, $5.4 billion dollars
of new biomedical construction came on line. Between 2003 and
2007, it is estimated that this figure will approximately double to $9.5
billion dollars of new research facilities (AAMC Survey of Research
Facility Investments: 99 out of 125 AAMC member schools; data
based on AAMC faculty roster). That such a large amount of the
growth in new research space is taking place after the end of the
doubling of the budget is simply a reflection of the time lag involved
in designing and building facilities. Along with this growth, however,
has come an increased demand for grant support, as new faculty
were hired and the institutions realized that each of these new
research facilities was mortgaged, to varying degrees, against future
indirect cost revenues. This mortgage, in turn, will fall to the NIH
Institutes and Centers to cover as part of the cost of future grants.
What Then Is Driving the NCI's Current Budget
Anxiety?
As Table 1 shows, the number of NCI Research Project Grant
(RPG) applicants who received awards peaked in 2004, with 1,393
grantees (new and competing renewals) receiving 1,487 grants. All
of this hoped-for growth in cancer research did not just slow down
and readjust to a new equilibrium when the growth of the annual
appropriation ended. Instead of a soft landing, the subsequent flat
budgets of 2004 through 2006 have brought the growth of the
cancer research enterprise to an uncomfortably sudden stop. The
outlook for the future includes a FY 2007 President's Budget with a
projected decrease of 0.8% from the 2006 level.1 This decreasing
budget is further eroded by a Biomedical Research and Development
Price Index (BRDPI) inflation rate of ~3.8% per year
(July 24, 2006 revision of BRDPI: Revised FY 2005 Update. Each
December or early January, the Bureau of Economic Analysis,
Department of Commerce, provides an estimate of the BRDPI for
the fiscal year completed on the previous September 30). This
number reflects changes in the cost of doing biomedical research and
commonly runs several points above the country's general inflationary
index. As shown in Fig. 1, there has been an 8.3% decrease in
the purchasing power of biomedical dollars from 2004 to 2007.
Table 1. Competing applicants and applications for FY 1995 to 2005
(data from NIH Office of Extramural Research)
FY |
Data on individuals
|
|
Data on applications |
All RPG
|
|
R01 Equivalent
|
All RPG |
|
R01 Equivalent |
| Reviewed |
Awarded |
Funded
(%) |
Reviewed |
Awarded |
Funded
(%) |
|
Reviewed |
Awarded |
Success
rate (%) |
|
Reviewed |
Awarded |
Success
rate (%) |
| 1995 |
3,095 |
838 |
27.1 |
2,773 |
665 |
24.0 |
3,671 |
870 |
23.7 |
3,190 |
685 |
21.5 |
| 1996 |
2,888 |
916 |
31.7 |
2,569 |
782 |
30.4 |
3,380 |
976 |
28.9 |
2,918 |
819 |
28.1 |
| 1997 |
2,970 |
951 |
32.0 |
2,645 |
802 |
30.3 |
3,520 |
993 |
28.2 |
3,048 |
826 |
27.1 |
| 1998 |
2,773 |
983 |
35.4 |
2,436 |
793 |
32.6 |
3,195 |
1,047 |
32.8 |
2,748 |
842 |
30.6 |
| 1999 |
3,289 |
1,165 |
35.4 |
2,722 |
888 |
32.6 |
3,878 |
1,244 |
32.1 |
3,093 |
932 |
30.1 |
| 2000
| 3,635 |
1,086 |
29.9 |
2,724 |
816 |
30.0 |
4,382 |
1,151 |
26.3 |
3,129 |
855 |
27.3 |
| 2001 |
3,666 |
1,126 |
30.7 |
2,836 |
827 |
29.2 |
4,374 |
1,188 |
27.2 |
3,254 |
856 |
26.3 |
| 2002 |
3,794 |
1,181 |
31.1 |
2,844 |
828 |
29.1 |
4,588 |
1,264 |
27.6 |
3,311 |
867 |
26.2 |
| 2003 |
4,251 |
1,326 |
31.2 |
3,172 |
943 |
29.7 |
5,249 |
1,421 |
27.1 |
3,745 |
996 |
26.6 |
| 2004 |
4,870 |
1,393 |
28.6 |
3,325 |
946 |
28.5 |
6,148 |
1,487 |
24.2 |
3,986 |
992 |
24.9 |
| 2005 |
5,050 |
1,226 |
24.3 |
3,480 |
812 |
23.3 |
6,325 |
1,292 |
20.4 |
4,147 |
844 |
20.4 |
|
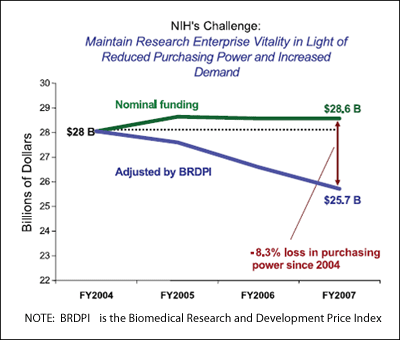
Figure 1. Biomedical research and development price index impact.
Figure 2 shows the NCI's Congressional appropriations from
1998 to 2006 and the President's Budget for 2007. The sidebar
shows the appropriation in constant dollars relative to 1998.
However, the greatest stress on a flat or decreasing budget comes
from the fact that >85% of each year's appropriated budget is
already committed to support the ''out years'' of previously awarded
grants and other ongoing activities.
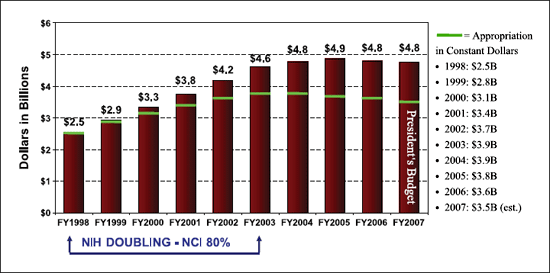
Figure 2. NCI's congressional appropriations, F7 1998 to FY 2007.
During the period from 1999 to 2005, the applicant pool for
grants increased by 53%, and application numbers increased by
63%. Presently, the amount of recycling within the grant pool is largely
based on grants awarded in 2001, when we were only a few
years into the doubling process. Thus, there is a lag period until the
recycle pool of grant awards catches up to the peak awards of 2004
at the completion of the doubling.
To get a better understanding of the strain on the current
budget, it is necessary to review the growth that NCI has
experienced - and continues to experience - in the number of
grant applicants and applications. As can be seen in Tables 2 and 3,
during the last 5 years of budget doubling (1999-2003), there were
a total of 962 new applicants. Between 2003 and 2005, there were
almost as many more new applicants (799).
| Table 2. NCI competing applicants |
| FY 1999 |
FY 2003 |
FY 2003 |
FY 2005 |
| 3,289 appl. |
4,251 appl. |
4,251 appl. |
5,050 appl. |
| Increase = 962 |
Increase = 799 |
Abbreviation: appl., applicants.
NOTE: The increase in competing applicants in the last 2 yrs is nearly as large as the increase in applicants during the period of NIH doubling.
|
| Table 3. NCI competing applications |
| FY 1999 |
FY 2003 |
FY 2003 |
FY 2005 |
| 3,878 appln. |
5,249 appln. |
5,249 appln. |
6,325 appln. |
| Increase = 1,371 |
Increase = 1,076 |
Abbreviation: appln., applications.
NOTE: The increase in competing applications in the last 2 yrs is nearly as large as the increase in applications during the period of NIH doubling.
|
The pattern of new competing applications is similar, with an
increase of 1,371 between 1999 and 2003 followed by an additional
increase of 1,076 between 2003 and 2005. This rapid increase in the
number of new applications has led to a continuing decline in the
success rate, the ratio of awards to the number of applications.
This indicator, which dropped from 32% to 27% between 1999 and
2003, was 19% in 2006, as the denominator continues to increase
with no indication yet of a plateau.2
During this same period, the number of awards averaged 1,276
per year (range, 1,119-1,494). The data also help to dispel several
common misperceptions, such as the belief that there has been a
greater increase in investment in solicited projects compared with
unsolicited R01s and P01s. A comparison of the percentage of
solicited to unsolicited grants between 1998 and 2005 (Fig. 3)2
shows these trends to be essentially unchanged over this time
period. The suggestion that there has been an increase in the
number of applications per investigator is also not supported by
the data, which indicate only a slight shift from 1.2 applications per
investigator to 1.3.
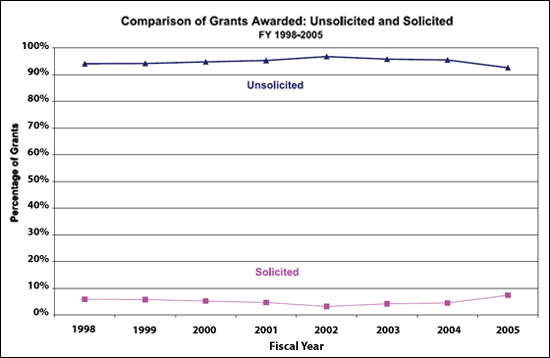
Figure 3. NCI's unsolicited RPGs versus solicited RPGs.
What Happens to the NCI Appropriation Once
Congress Passes the Budget?
In recent years, and again for FY 2007, it has been unusual for
Congress to complete the appropriations process for the budget
before the October 1 start of the new fiscal year. As a result, the NCI
often begins the year operating under a ''Continuing Resolution,''
with the available funding based on the prior year's budget. This
has a major effect on grantees, as resources are held back and only
a percentage of their grants are paid until the actual NCI
appropriation is determined.
Once there is a final NCI appropriation, a number of adjustments,
including a series of decreases and mandatory taps must be
made. The 2006 appropriation provides an excellent case in point.
Although the 2006 appropriation dollar figure has been published
and continues to be referred to as the ''annual budget,'' there was a
legislatively mandated across-the-board 1.0% decrease in the actual
discretionary budget before any other requests were considered.
With this reduced appropriation, the NCI then began to subtract
additional funds for mandatory needs.
On average, approximately $30 million (M) must be set aside to
address these needs, which include taps from the Department of
Health and Human Services and the NIH. Taps from the NIH meet
needs on campus, such as utility and rent increases and security
assessments. In 2006, for example, $6.8M was needed to support
national efforts, such as the ongoing aid to victims of Katrina and
staffing needs of the Centers for Medicare and Medicaid Services,
which is handling overwhelming beneficiary interest in its
Medicare Plan D benefit. There were also mandatory federal salary
increases in 2006, as well as the usual, annual ''out year''
commitments of approximately $1.6B for the noncompeting RPG
pool (the multi-year projects already under way and for which
funds are committed). The NCI's contribution for the NIH
Roadmap, a common fund that was created to help enable science
across disciplines and diseases, increased to $43M.3 The NCI's
investment in the Roadmap has paid dividends. Our investigators
compete well for the shared funds, and the Institute has benefited
from the trans-NIH collaborations this mechanism has enabled.
Nevertheless, Roadmap dollars still represent an upfront tap to the
appropriated budget that must be found in the fraction of
uncommitted funds and cannot be used to ease the current
budgetary pressures.
RPGs, which include among others P01, R01, R03, and R21
funding mechanisms for investigator-initiated research proposals,
account for 46% of the NCI budget, a percentage that has
remained fairly constant for at least the last 15 years. The
predominant grant mechanism comprising the RPGs is the R01.
The NIH established three major policy variables for RPGs for FY
2006. These included (a) that noncompeting grants be awarded
at a level 2.35% below the prior level of commitment and that
reduction be reflected on any future year commitments for that
grant, (b) that the average cost of competing RPGs be the same
in FY 2006 as it was in FY 2005, and (c) that the number of
competing RPGs in FY 2006 be around the same as in 2005. It is
anticipated that similar variables for RPGs for FY2007 will be
established by the NIH.
Furthermore, the NCI continues to make it a priority to fund
first-time investigators. There is agreement across the biomedical
community that the careers of young scientists must be nurtured.
The R29, a now-defunct grant mechanism, was introduced by the
NIH to exclusively fund young investigators. However, the size of
the award, less than half that of the standard R01 grant, as well
as the lack of value placed on R29 grantee status by universities,
led to its discontinuation. The NIH has also asked reviewers to
de-emphasize preliminary data on R01 applications from new
investigators, but this approach has also failed to increase the
proportion of NIH grantees under the age of 35.
The average age of the first-time NIH grantee continues to
increase. In 2002, a National Academy of Sciences panel was
charged by NIH director Dr. Elias Zerhouni to address this problem.
In keeping with some of the suggestions that emerged
from that panel, NCI instituted, in FY 2002, the Star (*)R01 program
for first-time R01 applicants. This program established a policy
of extending the regular R01 pay line by a variable number of
percentile points to consistently fund the same number of
competing first-time R01 investigators every year, ensuring that
the pipeline of cancer researchers of the future remains consistently
strong. In FY 2006, the NCI devoted approximately $18 M
to exceptions for *R01 investigators. After achieving their first
successful R01 grant award as a beginning faculty member, an
important factor in tenure/promotion decisions is the successful
competitive renewal of that first grant. In recognition of this
renewal as a critical step in establishing a research career, the NCI
is proposing to explore ways of fostering institutional support for
this important second step.
In past years, a Director's Reserve fund ($100-125 M) has been
set aside for start-up of scientific initiatives. In FY 2006, this fund
was reduced to $25 M which was used to cover emergency
assessments. It is anticipated that a Director's Reserve for FY2007
will be set aside at the same level for unexpected taps and
assessments.
Decisions regarding research support are appropriately well
grounded in an intensive peer review process for intramural and
extramural projects. Figure 4 shows the FY 2005 actual obligations
of funding by mechanisms.4 Nearly 80% of the NCI budget in FY
2005 was spent for extramural research activities. The intramural
research program, which includes basic science, epidemiology, and
clinical studies done at the NIH Clinical Research Center, currently
receives <9% of the annual budget for research activities.1 This
percentage is down from 10% in FY 2004. In overall dollars, the size
of the intramural program actually decreased, for example, from FY
2004 to FY 2006 by >3%. The NCI intramural research program in
Bethesda and Frederick, MD undergo rigorous peer review. This
downsizing, therefore, was carefully engineered with the significant
peer review guidance of the NCI's Board of Scientific Counselors.
The Board of Scientific Counselors are also providing leadership to
enhance intramural/extramural collaboration and integration
around scientific priorities. Yet, the NCI's component of the overall
NIH intramural research program has continued to thrive,
conducting high-risk and distinctive research, distributing technology,
training future scientists, and forging partnerships to the
benefit of cancer patients and the scientific community. The NCI as
a whole can do no less in the face of monetary challenges.
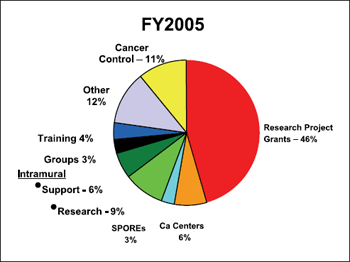
Figure 4. NCI budget allocation by mechanism in FY 2005.
NCI's Executive Committee Budget Planning
Process
During the past 2 years, the leadership of the NCI, its Executive
Committee, has reduced or phased out programs, allowing the
redeployment of resources to initiate new programs or to increase
support for existing programs. To maintain momentum toward the
Institute's scientific goals, the Executive Committee has had to
engage in a rigorous review of Division programs and make
difficult decisions concerning which projects should be maintained,
downsized, or eliminated in order to continue to make
strategic investments. This represents a return of the planning
process to the Division and Center Directors, all of whom are on
the Executive Committee.
Although the ''pay line'' is a number of considerable interest
to the research community, another major factor that drives the
budget decision-making process is the number of qualified
applicants and applicant institutions seeking support for unsolicited
research proposals, and the success rate they are experiencing.
The number of qualified applicants and applicant organizations
is a difficult denominator to project, and determining a reasonable
success rate to ensure the highest quality science directed at
cancer is even more problematic (Fig. 5).2 Nevertheless, this is what
the NCI must strive to quantify and attain as we plan future
budgets.
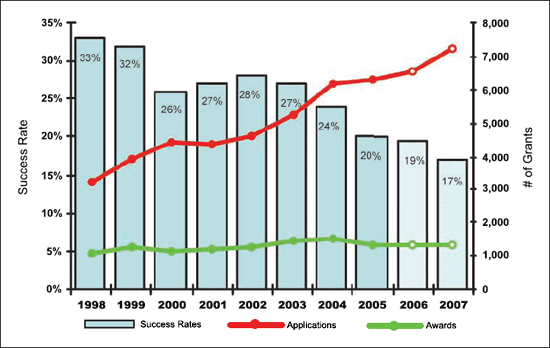
Figure 5. Applications, awards, and success rates: FY 1998 to FY 2007 estimate competing RPGs.
Planning for the 2007 budget began in the spring of 2006, with
an intensive two-day retreat of the Executive Committee in
which directors of the Divisions and Centers presented their
program portfolios and indicated their funding preferences for
2007. Executive Committee members evaluated programs based on
their respective priority assessments. The resulting priority assessments
were evaluated during subsequent meetings. This process,
which has involved a great deal of planning and preparation, is
providing the entire NCI leadership with an in-depth understanding
of the major initiatives within the Institute and, perhaps more
importantly, a clearer view of what can be done across Divisions to
achieve greater efficiencies in the use of resources. The sessions
have also provided an opportunity to strategically explore the
possibility of enhanced cross-Division scientific opportunities. This
scientific retreat was followed this summer and fall by a similar in-depth
review of the NCI's infrastructure support.
Throughout the year, the Executive Committee receives updates
on both scientific and administrative programs with an eye
towards monitoring progress and being prepared to adapt to
budgets that may be reduced or flat for the foreseeable future. In an
era of flat or deficit budgets, there is a natural hesitancy to commit
to long-term obligations. The key to making future commitments is
determining where the most worthwhile opportunities lie. During
these meetings, the Division and Center directors work as a
cohesive leadership group. I have been thoroughly impressed by
their ability to work together, and with extensive input from
external advisors, to make difficult decisions with a unified mission
to sustain scientific progress.
External Community Involvement Is Critically
Important
These decisions on resource allocation and prioritization are
not made in a vacuum. NCI leaders rely on guidance from our key
advisory boards, which provide scientific review of research
proposals and counsel on establishing priorities. This feedback -
during regularly scheduled meetings, such as subcommittee deliberations
and the annual January advisory boards retreat -
figures prominently in the Executive Committee's budget deliberations.
The Director and Division and Center heads have
historically had close relationships with the external scientific
community, through personal contacts and through their Program
Staff, who serve as the point of contact for extramural researchers to
the NCI. Additionally, there are numerous workshops, think
tanks, working groups, and scientific meetings sponsored each year
by the NCI that are widely attended by clinicians, researchers,
advocates, and others in the oncology community. These formal
and informal interactions provide not only insights into emerging
scientific opportunities, but also serve to expose other issues that
need airing.
The NCI is fortunate in the number of scientists, advocates,
and others who give of their time to serve the Institute on advisory
boards and ad hoc scientific committees, and by participating
in advocacy activities and disparities programs. The number of
people who come to the NCI each year to participate in these
various meetings and workshops is in the hundreds, if not
thousands. All of these activities - whether it is a review of an
intramural research program, a workshop on the state of the
science, a meeting on cancer control, or participation in an
advocacy summit - contribute to defining the scientific direction of
the Institute.
There are also opportunities for scientific input during meetings
of the Cancer Center directors and Specialized Programs of
Research Excellence principal investigators, as well as during the
American Association of Cancer Research and American Society of
Clinical Oncology annual meetings. Throughout each year, the NCI
is visited by various groups who come to Bethesda or Frederick,
first and foremost, to discuss science, but also to talk about
strategic direction, administrative and management issues, and
other topics that affect them. The NCI has always been willing to
meet with groups and carefully listen to their concerns and ideas
about resource allocation. In all situations, when a new scientific
initiative is considered, a group of experts is assembled to provide
appropriate advice.
The Future: Some Personal Thoughts
There is broad agreement among NCI leaders about our path
forward. First, we must continue to address the NCI's strategic
priorities by funding new initiatives. Second, we are committed to
striving to maintain the number of competing awards near the
level achieved by the doubling of the NIH budget. In addition, we
are firmly committed to funding new investigators. As a nation, we
need to continue to make biomedical research an attractive career
choice. It would be devastating if the best minds are kept away
from a field so vital to our nation's health and economics.
Discussing priority setting and budget planning in this and other
venues ensures greater transparency and openness. Hopefully, it
will have another outcome: to unify the voice of advocacy for
cancer research and care. Often fragmented, support for cancer
research is tied to individual cancer types or specific scientific
programs. To make the kind of impact that is needed today, the
cancer community must speak with a unified voice. We must
propose solutions as a community if we are to be effectively heard.
I have personally experienced major swings in biomedical
research funding over the past 30 years. Such swings can take
their toll on scientific momentum and discourage new researchers
from committing to a career in research (Fig. 6; ref. 1). I am urging
us, as a cancer research community, to speak for a national plan in
support of the biomedical research enterprise. I believe that such a
plan is needed to maintain the United States' position as a
worldwide scientific leader. The future of science and the economic
vigor of our country will depend on investment in the life sciences,
genetics, and biotechnology. We need to recognize the importance
of healthcare, of reducing mortality from disease, as a critical driver
of the country's economic welfare.
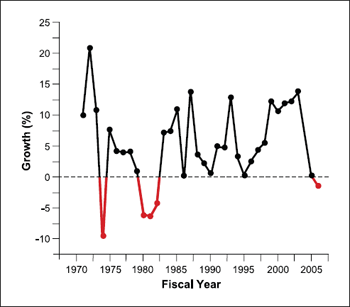
Figure 6. Annualized growth of the NIH budget, 1971 to 2005.
As Director of the NCI, I am working hard to find new ways to
leverage resources. We are continuing our collaborations with other
NIH Institutes and Centers and Federal agencies. NCI has
long-standing partnerships and collaborations, such as the
Surveillance, Epidemiology, and End Results program with the
Centers for Disease Control and Prevention and the National
Program of Cancer Registries; the Interagency Oncology Task Force
with the Food and Drug Administration; and Cancer Control
PLANET with the Agency for Healthcare Research and Quality, the
Substance Abuse and Mental Health Services Administration, and
the Health Resources and Services Administration within the
Department of Health and Human Services. The Surveillance,
Epidemiology, and End Results program and Cancer Control
PLANET are also supported by the American Cancer Society. Each
of the Divisions and Centers at NCI has been extraordinarily
successful in developing collaborations with academia, other
Federal agencies, and other NIH Institutes and Centers. For example,
NCI cofunds programs across the oncology research
spectrum in behavioral science, clinical research, epidemiology,
genetics, molecular biology, proteomics, nanotechnology, and basic
scientific discovery, just to name a few. I also plan to increase
public-private partnerships during my tenure at NCI.
Aside from the logical hand-offs in drug development for new
oncology drugs and biologics coming out of the intramural
translational research program, the NCI has unique capabilities
on the Frederick Campus. The technology expertise, core facilities,
and advanced biomedical technology development opportunities
at NCI-Frederick are a unique national resource. The NCI Research
Technology Program is home to advanced technologies in imaging,
analytic and protein chemistry, genomics, structural biology,
and supercomputing capabilities. When the NCI undertakes a
new initiative such as large-scale technology-based programs,
intramural and extramural experts are asked to help advise and
plan the program with NCI staff. Advice is sought through
think tanks, working groups, state-of the-science meetings and
advisory board ad hoc subcommittees. Additionally, advisory
board approval is obtained prior to implementation. I am
committed to NCI's continuing leadership in developing enabling
technologies, such as bioinformatics, nanotechnologies, and The
Cancer Genome Atlas (a comprehensive description of the genetic
basis of human cancer), and the necessity of making these and other
resources accessible to the entire cancer research community.
There is no other organization or institution that can fill this need.
Although it is important to be realistic about the possibility of any
influx of Federal discretionary dollars flowing into the NCI soon, I
continue to be optimistic because I have seen what the cancer
research community is capable of accomplishing, regardless of the
obstacles presented. By the mid-1990s, the inexorable climb of
cancer mortality rates, a trend since the first mortality statistics
were compiled in 1930, was finally halted and reversed. This
downward trend has now been sustained for over a decade, and
through the efforts of our community, can not only continue, but
accelerate (2).
Our responsibilities at the NCI and as a cancer community are to
continue conducting quality research, to make biomedical research
an attractive career choice, to offer solutions to our challenges, to
speak with a more unified voice, and to make the difficult decisions
among competing priorities that will be necessary to maintain our
momentum. The NCI remains steadfastly committed to the RPG
pool as its highest priority. As evidenced by the advances and
progress recently made in cancer mortality, our past investment
has paid dividends. These are exciting times in which science must
flourish, but we must continue to foster and nourish our
opportunities. We cannot stand on our laurels; we cannot maintain
the status quo. The NCI will do its part to foster and enable new
discoveries, new technologies, and the development of the
scientific leaders of tomorrow. It is up to others, with the reminder
that one in two men and one in three women will receive a
diagnosis of cancer in their lifetime, to ensure that NCI has the
resources and the authority to capitalize on the recent, historical
decline in the number of people dying from cancer each year.
References
1. Loscalzo J. The NIH budget and the future of biomedical research. N Engl J Med
2006;354:1665-7.
2. Ries LAG, Harkins D, Krapcho M, et al., editors. SEER Cancer Statistics Review,
1975-2003. Bethesda (MD): NCI.
Notes
1At the time of publication, the FY 2007 budget was under a Continuing Resolution.
It has been suggested that the budget may be under Continuing Resolution for the
entire year. However, since it is not known, this article contains the President's Budget
for FY 2007.
2NCI Office of Budget and Financial Management data.
3NCI Participation in Trans-NIH Strategic Initiatives at http://www.cancer.gov/
researchandfunding/NIHRoadmap/page2.
4National Cancer Institute 2005 Fact Book. NIH Publication no. 06-0512, page v.
Back to Top |