Short title: Prophylactic natural surfactant
Reviewer(s): Soll RF
Date of most recent amendment: 28/08/1997
Date of most recent substantive amendment: 27/08/1997
Date next stage expected: / /
Contact:
Dr Roger F. Soll MD
Associate Professor of Pediatrics
Department of Pediatrics
University of Vermont College of Medicine
A-121 Medical Alumni Building
Burlington, VT
USA
05405-0068
Telephone1: +1-802-656-2392
Telephone2:
Facsimile: +1-802-656-2077
E-mail: rsoll@salus.med.uvm.edu
Sources of support for the review
Neonatal Collaborative Review Group, NIH Contract N01-MD-6-3253
Acknowledgements
I would like to thank Nancy Moreland for preparation of the
manuscript.
Conflict of interest
Dr. R. Soll has acted as a consultant and invited speaker
for several of the pharmaceutical companies which manufacture
surfactant preparations (Abbott Laboratories, Ross Laboratories,
Chiesi Pharmaceuticals, Dey Laboratories, Burroughs-Wellcome).
To assess the effect of prophylactic intratracheal administration of natural surfactant extract in preterm newborns at risk for developing respiratory distress syndrome (RDS).
Searches were made of the Oxford Database of Perinatal Trials, Medline (MeSH terms: pulmonary surfactant; limits: age groups; newborn infants), previous reviews including cross references, abstracts, conference and symposia proceedings, expert informants and journal hand searching in the English language.
Randomized controlled trials which compared the effect of prophylactic natural surfactant administration (surfactant obtained from human or bovine sources, either modified with additional phospholipids or not) administered to high risk preterm newborns at or shortly after birth in order to prevent respiratory distress syndrome, other complications of prematurity, and mortality.
Data regarding clinical outcomes including incidence of pneumothorax, pulmonary interstitial emphysema, patent ductus arteriosus, necrotizing enterocolitis, intraventricular hemorrhage (any grade and severe intraventricular hemorrhage), bronchopulmonary dysplasia, mortality, bronchopulmonary dysplasia or death, and retinopathy of prematurity were excerpted from the reports of the clinical trials by the reviewer. Data analysis was done in accordance with the standards of the Cochrane Neonatal Review Group.
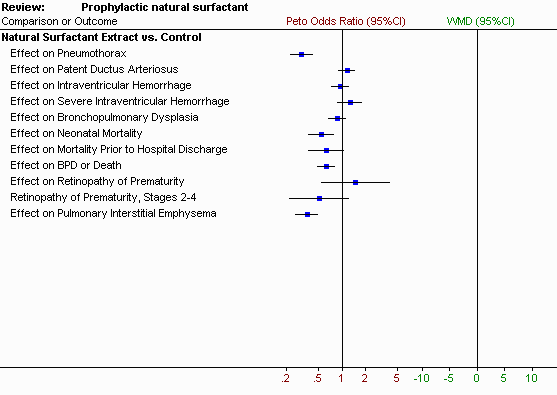
All of the included studies note an initial improvement in respiratory status and a decrease in the risk of respiratory distress syndrome in infants who receive prophylactic natural surfactant extract. The meta-analysis supports a decrease in the risk of pneumothorax (typical relative risk 0.35, 95% CI 0.26, 0.49; typical risk difference -0.15, 95% CI -0.20, -0.11), a decrease in the risk pulmonary interstitial emphysema (typical relative risk 0.46, 95% CI 0.35, 0.60; typical risk difference -0.19, 95% CI -0.25, -0.13), a decrease in the risk of neonatal mortality (typical relative risk 0.60, 95% CI 0.44, 0.83; typical risk difference -0.07, 95% CI -0.12, -0.03), and a decrease in the risk of bronchopulmonary dysplasia or death (typical relative risk 0.84, 95% CI 0.75, 0.93; typical risk difference -0.10, 95% CI -0.16, -0.04. No differences are reported in the risk of intraventricular hemorrhage, patent ductus arteriosus, necrotizing enterocolitis or retinopathy of prematurity. Few data are available on long-term follow-up of treated infants.
Prophylactic intratracheal administration of natural surfactant extract to infants judged to be at risk of developing respiratory distress syndrome (intubated infants <30 weeks gestation) has been demonstrated to improve clinical outcome. Infants who receive prophylactic natural surfactant extract have a decreased risk of pneumothorax, a decreased risk of pulmonary interstitial emphysema, a decreased risk of mortality, and a decreased risk of bronchopulmonary dysplasia or death.
Respiratory distress syndrome (RDS) is caused by a deficiency or dysfunction of pulmonary surfactant. Surfactant lines the alveolar surface and prevents atelectasis at end expiration. Pulmonary surfactant is predominantly dipalmitoylphosphatidylcholine with lesser amounts of other phospholipids including phosphatidylglycerol (PG), phosphatidylethanolamine, and phosphatidylinositol. Pulmonary surfactant also contains neutral lipids and distinct surfactant proteins. The physiologic functions of surfactant include the ability to lower surface tension, and the ability to rapidly adsorb, spread and reform a monolayer in the dynamic conditions associated with the respiratory cycle.
Investigators in the 1960s attempted to aerosolize dipalmitoylphosphatidylcholine (DPPC) to infants with established respiratory distress syndrome. These investigators could not demonstrate any beneficial effect of surfactant replacement. The poor results were, in part, due to an incomplete understanding of what constitutes pulmonary surfactant. The first successful animal model of surfactant replacement therapy was conducted by Enhorning and coworkers (1972). Enhorning administered a crude natural surfactant extract obtained from lavage of the lungs of mature rabbits directly into the trachea of immature rabbits. Improvement in lung compliance and alveolar expansion was noted. Success in animal models led to widespread clinical trials in the newborn.
A wide variety of surfactant products have been formulated and studied in clinical trials. These include synthetic surfactants and natural surfactant extracts. Natural surfactant extracts are derived from animal or human sources. Natural surfactant extracts can be further classified as either modified or unmodified surfactant extracts; modified natural surfactant extract is supplemented with phospholipids or other surface active material while unmodified natural surfactant extract contains only the components remaining after the extraction process.
Trials of prophylactic administration of natural surfactant extract attempt to identify infants at high risk of developing respiratory distress syndrome. In these studies, infants were randomized to receive surfactant or control treatment immediately after delivery either prior to the onset of respiratory symptoms or within 15 minutes of birth. These investigators hoped to assure more homogeneous distribution of surfactant and decreased barotrauma which can occur with even short periods of ventilation (Jobe 1984, Nilsson 1978).
The following analysis is a systematic review of the 8 randomized controlled trials which compare the prophylactic administration of natural surfactant extract to control treatment.
To assess the effect of prophylactic intratracheal administration of natural surfactant extract in preterm newborns at risk for developing respiratory distress syndrome (RDS).
Types of studies: Randomized controlled trials comparing prophylactic natural surfactant extract administration (surfactant given down the endotracheal tube prior to the first breath or immediately after delivery room intubation and stabilization) to control treatment.
Types of participants: Premature infants gestational age < 30 weeks with or without evidence of surfactant deficiency.
Types of interventions: Infants randomized to receive prophylactic natural surfactant administration (pre-ventilatory or post-ventilatory) versus control treatment (intratracheal administration of normal saline or air placebo). All included studies utilized surfactant products derived from mammalian sources (human amniotic fluid extract, calf lung surfactant extract, or modified bovine surfactant extract).
Types of outcome measures: Data for the following clinical
outcomes are included in the meta-analysis:
1) pneumothorax
2) pulmonary interstitial emphysema
3) patent ductus arteriosus
4) necrotizing enterocolitis
5) intraventricular hemorrhage
6) severe intraventricular hemorrhage
7) bronchopulmonary dysplasia
8) retinopathy of prematurity
9) retinopathy of prematurity, stage 2-4
10) neonatal mortality
11) mortality prior to hospital discharge
12) bronchopulmonary dysplasia or death
Searches were made of the Oxford Database of Perinatal Trials, Medline (MeSH terms: pulmonary surfactant; limits: age groups; newborn infants), previous reviews including cross references, abstracts, conference and symposia proceedings, expert informants, and journal hand searching in the English language.
For each included study, information was collected regarding the method of randomization, blinding, drug intervention, stratification, and whether the trial was single or multicenter. Information regarding trial participants including gestational age criteria, birthweight criteria, and other inclusion or exclusion criteria was noted. Information on clinical outcome was analyzed including pneumothorax, pulmonary interstitial emphysema, patent ductus arteriosus, necrotizing enterocolitis, intraventricular hemorrhage (any intraventricular hemorrhage and severe intraventricular hemorrhage), bronchopulmonary dysplasia, retinopathy of prematurity, neonatal mortality, mortality prior to hospital discharge, and bronchopulmonary dysplasia or death.
Studies included in this review: Enhorning (1985), Kwong (1985), Merritt (1986), Kendig (1988), Soll (1990), Hoekstra (1991), Dunn (1991), and Shennan (1989). Details of each study are given in the "Characteristics of Included Studies" table and references.
All studies attempted to include infants thought to be at risk of developing respiratory distress syndrome, though entry criteria differ between the studies. All 8 studies sought to enroll infants <30 weeks gestation, although the specific gestational age criteria differ slightly. Enhorning (1985) and Dunn (1991) include infants <30 weeks gestation. Kwong (1985) included infants between 24 and 28 weeks gestation. Merritt (1986) included infants between 24 and 29 weeks gestational age. Kendig (1988) included infants between 25 and 29 weeks gestational age. Soll (1990) included infants between 24 and 30 weeks gestational age, and Hoekstra (1991) included infants between 23 and 29 weeks gestation. Shennan (1989) included all infants less than 29 weeks gestation. Merritt (1986), Hoekstra (1991) and Dunn (1991) excluded infants with evidence of lung maturity. Kwong (1985) excluded infants who had received >24 hours of antenatal steroid therapy. All studies attempted to exclude infants who were diagnosed as having major congenital anomalies.
Studies either administered the prophylactic natural surfactant prior to onset of the initiation of respiration (Enhorning 1985, Kwong 1985, Kendig 1988, Dunn 1991 and Shennan 1989), or administered surfactant in the delivery room immediately after intubation and stabilization (Merritt 1986, Soll 1990, and Hoekstra 1991).
In all of the studies, the surfactants used were natural surfactant extracts. Enhorning (1985), Kwong (1985), Kendig (1988), Dunn (1991), and Shennan (1989) all utilized a calf lung surfactant extract (CLL, CLSE, or BLSE). Merritt (1986) utilized a surfactant obtained from human amniotic fluid. Soll (1990) and Hoekstra (1991) utilized a modified bovine surfactant extract (Survanta).
Study outcomes included initial respiratory status, the incidence of respiratory distress syndrome, and a variety of complications of prematurity including pneumothorax, pulmonary interstitial emphysema, patent ductus arteriosus, necrotizing enterocolitis, intraventricular hemorrhage, retinopathy of prematurity, bronchopulmonary dysplasia, and mortality.
Randomized controlled trials which compare the effect of prophylactic natural surfactant administration (surfactant given down the endotracheal tube prior to the first breath or immediately after intubation and stabilization in the delivery room) compared to control treatment (sham air treatment or instillation of normal saline) to premature infants thought to be at risk for developing respiratory distress syndrome are included in the analysis. The eight included studies were of high methodologic quality. Specific methodologic issues are discussed below:
Randomization: All included studies allocated assigned treatment by randomization. In 7 of the studies, sealed envelopes with randomly allocated treatment assignments were provided to participating centers. In the study of Kendig (1988) coded vials were used for randomization.
Blinding of Treatment: Investigators attempted to blind treatment. Most studies relied on a resuscitation team to administer the randomly allocated treatment. Individuals in this resuscitation team were not responsible for ongoing care of the infant or for study evaluation.
Blinding of Outcome Assessment: Investigators who were not involved with treatment assignment or administration assessed the study outcomes.
Exclusion after Randomization: Minimal exclusions were noted after randomization. Kwong (1985) had a high number of exclusions because infants were enrolled prior to delivery and subsequently excluded if mothers had completed 24 hours of antenatal steroid therapy. A significant number were also excluded by Kwong (1985) if they were felt to be outside of the gestational age limits of the study.
Prophylactic intratracheal administration of natural surfactant extract in preterm infants at risk for developing RDS improves oxygenation (improved alveolar-arterial oxygen difference, improved arterial/alveolar oxygen ratio, decreased inspired oxygen concentration) and ventilation (decreased mean airway pressure, improved ventilator efficiency index) during the first 48-72 hours of life. Prophylactic intratracheal administration of natural surfactant extract in preterm infants at risk of developing RDS also had the following clinical impact:
Pneumothorax: Four of the randomized controlled trials reported a decreased incidence of pneumothorax associated with prophylactic surfactant administration. The typical estimate from the meta-analysis suggests that prophylactic administration of natural surfactant extract will lead to a significant reduction in the risk of pneumothorax (typical relative risk 0.35, 95% CI 0.26, 0.49; typical risk difference -0.15, 95% CI -0.20, -0.11).
Pulmonary Interstitial Emphysema: Five of the randomized controlled trials reported on the incidence of pulmonary interstitial emphysema. Four of these trials noted a significant reduction in the incidence of pulmonary interstitial emphysema associated with prophylactic surfactant administration. The typical estimate from the meta-analysis suggests that prophylactic administration of surfactant will lead to a significant reduction in the risk of pulmonary interstitial emphysema (typical relative risk 0.46, 95% CI 0.35, 0.60; typical risk difference -0.19, 95% CI -0.25, -0.13).
Patent Ductus Arteriosus: None of the individual trials reported a difference in the risk of patent ductus arteriosus. The typical estimate of the meta-analysis supports no difference in the risk of patent ductus arteriosus (typical relative risk 1.08, 95% CI 0.94, 1.24; typical risk difference 0.03, 95% CI -0.03, 0.09).
Intraventricular Hemorrhage: Enhorning (1985) noted a decrease in the incidence of intraventricular hemorrhage in infants who received prophylactic natural surfactant administration (risk difference -0.324, 95% CI -0.543, -0.106). The typical estimate from the meta-analysis suggests that prophylactic administration of natural surfactant extract does not influence the risk of intraventricular hemorrhage (typical relative risk 0.98, 95% CI 0.84, 1.15; typical risk difference -0.01 95% CI -0.07, 0.05).
Severe Intraventricular Hemorrhage: None of the individual trials support a difference in the incidence of severe intraventricular hemorrhage (Grade III or IV intraventricular hemorrhage). The typical estimate of the meta-analysis supports no difference in the risk of severe intraventricular hemorrhage (typical relative risk 1.22, 95% CI 0.90, 1.66; typical risk difference 0.03, 95% CI -0.02, 0.07).
Bronchopulmonary Dysplasia: None of the individual trials support a difference in the incidence of bronchopulmonary dysplasia in all treated infants (not just survivors). For the purpose of these studies, bronchopulmonary dysplasia was defined as an oxygen requirement at 28 days of age. The typical estimate of the meta-analysis supports no difference in the risk of bronchopulmonary dysplasia (typical relative risk 0.93, 95% CI 0.80, 1.07; typical risk difference -0.03, 95% CI -0.09, 0.03).
Mortality: Three of the randomized controlled trials report a decrease in neonatal mortality. The studies of Enhorning (1985), Merritt (1986) and Hoekstra (1991) all support a decrease in the incidence of neonatal mortality associated with the administration of prophylactic natural surfactant extract. The typical estimate from the meta-analysis suggests that prophylactic administration of natural surfactant extract leads to a significant reduction in the risk of neonatal mortality (typical relative risk 0.60, 95% CI 0.44, 0.83; typical risk difference -0.07, 95% CI -0.12, -0.03).
Six of the randomized controlled trials reported on mortality prior to hospital discharge. Enhorning (1985) and Merritt (1986) reported a decrease in the incidence of mortality prior to hospital discharge. The typical estimate from the meta-analysis supports no difference in the risk of dying prior to hospital discharge (typical relative risk 0.70, 95% CI 0.47, 1.06; typical risk difference -0.06, 95% CI -0.14, 0.01).
Bronchopulmonary Dysplasia or Death: Seven of the randomized controlled trials reported the combined outcome of bronchopulmonary dysplasia or death. Three of the randomized controlled trials supported a decrease in this combined outcome. The typical estimate from the meta-analysis suggests that prophylactic administration of natural surfactant extract will lead to a significant reduction in the risk of bronchopulmonary dysplasia or death at 28 days (typical relative risk 0.84, 95% CI 0.75, 0.93; typical risk difference -0.10, 95% CI -0.16, -0.04).
Retinopathy of Prematurity: Five of the randomized controlled trials reported on retinopathy of prematurity. Kwong (1985) and Kendig (1988) reported on infants with any stage of retinopathy. Enhorning (1985), Shennan (1989), and Dunn (1990) only reported those infants with Stage II disease or greater. No individual trial reported a difference in the incidence of retinopathy of prematurity and the meta-analysis supports no difference in the risk of retinopathy of prematurity associated with prophylactic administration of natural surfactant extract. The meta-analysis suggests that prophylactic surfactant administration does not lead to differences in the risk of any retinopathy (typical relative risk 1.37, 95% CI 0.63, 2.98; typical risk difference 0.07, 95% CI -0.10, 0.23) or retinopathy Stage 2-4 (typical relative risk (0.58, 95% CI 0.27, 1.24; typical risk difference -0.05, 95% CI -0.12, 0.02).
Eight randomized controlled trials were identified which compared prophylactic administration of natural surfactant extract to control treatment. Studies used either calf lung or bovine lung surfactant extract (Enhorning 1985, Kwong 1985, Kendig 1988, Dunn 1990, and Shennan 1989), modified bovine surfactant extract (Soll 1990, Hoekstra 1991) or human amniotic fluid extract (Merritt 1986). All trials enrolled high risk infants identified on the basis of gestational age. All infants studied were <30 weeks gestation, although the specific gestational age criteria differ slightly between studies. Kwong (1985) did not include any infants exposed to more than 24 hours of antenatal steroids. Only Hoekstra (1991) allowed for multiple treatment with surfactant.
Prophylactic administration of natural surfactant extract in preterm infants at risk for developing RDS led to improvement in oxygenation and ventilatory requirements in the 48-72 hours after treatment.
The meta-analysis suggests that prophylactic administration of natural surfactant extract leads to a significant decrease in the incidence of pneumothorax, pulmonary interstitial emphysema, neonatal mortality, and the incidence of bronchopulmonary dysplasia or death. The meta-analysis suggests that for every 100 infants treated prophylactically there will be 15 fewer pneumothoraces, 19 fewer cases of pulmonary interstitial emphysema, and 7 fewer neonatal deaths. No impact is noted on the incidence of intraventricular hemorrhage or severe intraventricular hemorrhage.
A previous review reported a small increase in pulmonary hemorrhage associated with use of exogenous surfactants (Raju 1993). This complication may in fact be hemorrhagic pulmonary edema secondary to massive ductal shunting. This outcome was not addressed in the initial trials, so the estimate of this effect was not reported in this meta-analysis. In clinical practice, pulmonary hemorrhage may be preventable by treatment of the ductus arteriosus and appropriate ventilatory management. No other side effects of surfactant treatment have been reported.
The trials included in this review compared prophylactic natural surfactant with no surfactant treatment. After the demonstration of the efficacy of surfactant in preventing and/or treating RDS, trials were conducted which compared the policies of prophylactic surfactant administration in babies at risk of RDS with selective surfactant treatment of babies who develop RDS. These trials showed that prophylactic surfactant may be superior to the later, selective treatment of babies with established RDS (see review by Soll RF, Morley CJ: Prophylactic Surfactant vs. Treatment with Surfactant).
Prophylactic administration of natural surfactant extract to infants judged to be at risk for developing respiratory distress syndrome has been demonstrated to improve clinical outcome. Infants who received prophylactic natural surfactant have a decreased incidence of respiratory distress syndrome, a decreased incidence of pneumothorax, a decreased incidence of pulmonary interstitial emphysema, a decreased incidence of neonatal mortality, and a decreased incidence of bronchopulmonary dysplasia or death. Surfactant treatment may lead to an increase in the incidence of pulmonary hemorrhage.
Prophylactic administration of natural surfactant extract has been proven to improve clinical outcomes. Further placebo controlled trials of prophylactic natural surfactant extract are no longer warranted. Trials have been conducted which compare the prophylactic administration of natural surfactant extract to selective treatment with natural surfactant extract (see review: "Prophylactic Surfactant versus Treatment with Surfactant"). The impact of prophylactic synthetic surfactant administration is discussed in other reviews ("Prophylactic Administration of Synthetic Surfactant").
Study: Dunn 1991
Methods: Randomized
Single center
Blinding of randomization: yes (sealed envelopes)
Blinding of intervention: no
Complete follow-up: yes
Blinding of outcome measurement:
Stratification based on gestational age (24-26 weeks, 27-29 weeks)
and antenatal steroid exposure
Participants: Premature infants
Gestational age <30 weeks
ROM <2 weeks
No major congenital anomaly
No evidence of lung maturity
Interventions: Prophylactic bovine lung surfactant extract
(75-100 mg) vs selective administration of bovine lung surfactant
extract (100 mg/kg) in intubated infants with respiratory distress
less than 6 hours of age vs. sham treatment (air)
Infants receiving surfactant were eligible for 3 additional doses
Outcomes: PRIMARY OUTCOME: a/A ratio
SECONDARY OUTCOME: ventilatory requirements
duration of assisted ventilation
complications of prematurity
Study: Enhorning 1985
Methods: Randomized
Single center study
Blinding of Randomization: yes (sealed envelopes)
Blinding of Intervention: attempted (staff not involved with clinical
care for next 5-6 days after administered treatment)
Complete Follow-up: yes
Blinding of outcome measurement: attempted
Stratification based on gestational age and exposure to antenatal
steroids
Participants: Premature Infants
Gestational age <30 weeks
Interventions: Prophylactic BLSE (75-100 mg) vs. control
(sham air instillation) via endotracheal tube
(Given prior to initiation of respiration)
Outcomes: PRIMARY OUTCOME: improvement in a/A ratio
duration of oxygen
administration
SECONDARY OUTCOMES:
duration of ventilation
complications of prematurity
Study: Howkstra 1991
Methods: Randomized
Multicenter study
Blinding of randomization: yes (sealed envelopes)
Blinding of intervention: attempted
Complete follow-up: yes
Blinding of outcome measurement: yes
Stratification based on birthweight and antenatal steroid exposure
Participants: Premature infants
Gestational age 23-29 weeks
Birthweight 600-1250 grams
Intubation and stabilization within 15 minutes after birth
No major congenital anomaly
No evidence of lung maturity
Interventions: Modified bovine surfactant extract (Survanta
100 mg/kg) vs. Sham treatment (air)
Given via endotracheal tube within 15 minutes of intubation and
stabilization
Infants allowed up to 3 subsequent doses if requiring assisted
ventilation and supplemental oxygen >30%
Outcomes: PRIMARY OUTCOME: death or bronchopulmonary dysplasia
at 28 days of age
SECONDARY OUTCOME:
respiratory status at 72 hours
incidence of respiratory distress
syndrome
complications of prematurity
Study: Kendig 1988
Methods: Randomized
Single center study
Blinding of randomization: yes (coded vials)
Blinding of intervention: yes
Complete follow-up: yes
Blinding of outcome measurement: yes
Participants: Premature infants
Gestational age 25-29 weeks
Required intubation at birth
Interventions: Prophylactic calf lung surfactant extract
(3 ml=90 mg) vs. normal saline (3 ml)
Given via endotracheal tube prior to initiation of respiration
Outcomes: PRIMARY OUTCOME: severity of respiratory distress
syndrome
SECONDARY OUTCOMES: physiologic variables including mean airway
pressure, ventilatory index, supplemental oxygen, radiographic
findings, complications of prematurity.
Study: Kwong 1985
Methods: Randomized
Single center study
Blinding of randomization: yes (opaque coded tubes)
Blinding of intervention: yes
Complete follow-up: no
Blinding of outcome measurement: yes
Participants: Premature infants
Gestational age 24-28 weeks
Antenatal steroids <24 hours
No major congenital anomaly
Interventions: Prophylactic CLSE (3 ml=90 mg) vs. normal
saline (3 ml) given via endotracheal tube prior to initiation
of respiration
Outcomes: PRIMARY OUTCOME: score based on radiographic
findings, requirement for supplemental oxygen, mean airway pressure,
ventilatory rate (IMV), ventilator efficiency index
SECONDARY OUTCOMES: complications of prematurity
Study: Merritt 1986
Methods: Randomized
Two participating centers
Blinding of randomization: yes (sealed envelopes)
Blinding of intervention: attempted
Complete follow-up: yes
Blinding of outcome measurement: yes
Participants: Premature infants
Gestational age 24-29 weeks
Lecithin/Sphingomyelin ratio <2
Phosphaidyl glycerol absent
No malformations known to influence fetal lung development
Interventions: Human surfactact extract (3 ml=60 mg) vs
sham treatment (air)
Given via endotracheal tube immediately after intubation and auscultation
to confirm appropriate endotracheal tube placement
Outcomes: PRIMARY OUTCOMES: clinical status at 28 days
(survival without bronchopulmonary dysplasia, survival with bronchopulmonary
dysplasia, death)
SECONDARY OUTCOMES: physiologic variables, requirement for respiratory
support, complications of prematurity.
Study: Shennan 1989
Methods: Randomized
Single center
Blinding of randomization: can't tell
Blinding of intervention: can't tell
Complete follow-up: can't tell
Blinding of outcome measurement: can't tell
Participants: Premature infants
Gestational age <29 weeks
Interventions: Prophylactic bovine lung surfactant extract
vs. sham treatment (air)
Outcomes: incidence of respiratory distress syndrome
complications of prematurity
Study: Soll 1990
Methods: Randomized
Multicenter study
Blinding of randomization: yes (sealed envelopes)
Blinding of intervention: attempted
Complete follow-up: yes
Blinding of outcome measurement: yes
Stratification by
Participants: Premature infants
Gestational age 24-30 weeks
Birthweight 750-1250 grams
Intubated and stabilized within 15 minutes of birth
No major congenital anomalies
Infants with proven sepsis excluded
Interventions: Modified bovine surfactant extract (survanta,
100 mg/kg) vs. sham treatment (air) Given via endotracheal tube
within 15 minutes of intubation and stabilization
Outcomes: PRIMARY OUTCOME: average change in mean
airway pressure and a/A ratio during 72 hours after treatment
SECONDARY OUTCOMES: clinical status at 7 and 28 days
radiographic finding at 24 hours
complications of prematurity
Dunn M, Shennan A, Possmayer F. Bovine surfactant prophylaxis in neonates less than 30 weeks' gestation: A Randomized controlled trial of prophylaxis versus treatment. Pediatrics 1991;87:377-386.
Enhorning G, Shennan A, Possmayer F, Dunn M, Chen CP, Milligan J. Prevention of neonatal respiratory distress syndrome by tracheal instillation of surfactant: a randomized clinical trial. Pediatrics 1985; 76:145-153.
Hoekstra RE, Jackson JC, Myers TF, Frantz ID, Stern ME, Powers WF, Maurer M, Raye JR, Carrier ST, Gunkel JH, Gold AJ. Improved neonatal survival following multiple doses of bovine surfactant in very premature neonates at risk for respiratory distress syndrome. Pediatrics 1991; 88:10-18.
Kendig JW, Notter RH, Cox C, Aschner JL, Benn S, Bernstein RM, Hendricks-Munoz K, Maniscalco WM, Metlay LA, Phelps DL, Sinkin RA, Woo9d BP, Shapiro DL. Surfactant replacement therapy at birth: final analysis of a clinical trial and comparisons with similar trials. Pediatrics 1988; 82:756-762.
Kwong MS, Egan EA, Notter RH, Shapiro DL. Double-blind clinical trial of calf lung surfactant extract for the prevention of hyaline membrane disease in extremely premature infants. Pediatrics 1985; 76:585-592.
Merritt TA, Hallman M, Bloom BT, Berry C, Benirschke K, Sahn D, Key T, Edwards D, Jarvenpaa AL, Pohjavuori M, Kankaanpaa K, Kunnas M, Paatero H,Rapola J, Jaaskelainen J. Prophylactic treatment of very premature infants with human surfactant. N Engl J Med 1986; 315:785-790.
Shennan A, Dunn M. Surfactant replacement trials - the need to maintain concurrent placebo controls. Pediatr Res 1989; 25:231A.
Soll RF, Hoekstra RE, Fangman JJ, Corbet AJ, Adams JM, James LS, Schulze K, Oh W, Roberts JD, Dorst JP, Dramer SS, Gold AJ, Zola EM, Horbar JD, McAuliffe TL, Lucey JF, Ross Collaborative Surfactant Prevention Study Group. Multicenter trial of single-dose modified bovine surfactant extract (Survanta) for prevention of respiratory distress syndrome. Pediatrics 1990; 85:1092-1102.
Enhorning G, Robertson B: Lung expansion in the premature rabbit fetus after tracheal disposition of surfactant. Pediatrics 1972;50:58-66.
Jobe A, Ikegami M, Jacobs M, Jones S. Surfactant and pulmonary blood flow distributions following treatment of premature lambs with natural surfactant. J Clin Invest 1984;73:848-856.
Nilsson R, Grossman G, Robertson B. Lung surfactant and the pathogenesis of neonatal bronchiolar lesions induced by artificial ventilation. Pediatr Res 1978;12:249-255.
Raju TN, Langenberg P: Pulmonary hemorrhage and exogenous surfactant. J Pediatr 1993;123(4):603-610.
01.00.00 Natural surfactant extract vs. control
01.01.00 Effect on pneumothorax
01.02.00 Effect on patent ductus arteriosus
01.03.00 Effect on intraventricular hemorrhage
01.04.00 Effect on severe intraventricular hemorrhage
01.05.00 Effect on bronchopulmonary dysplasia
01.06.00 Effect on neonatal mortality
01.07.00 Effect on mortality prior to hospital discharge
01.08.00 Effect on BPD or death
01.09.00 Effect on retinopathy or prematurity
01.10.00 Retinopathy or prematurity, stages 2-4
01.11.00 Effect on pulmonary interstitial emphysema
