Dystonia Protein Linked to Problem Common in Other Neurological Disorders
Skip secondary menuFor release: Monday, March 24, 2003

A new study links the protein that is impaired in the movement disorder torsion dystonia to a problem that is common to many neurological diseases. The finding may point to new treatments for dystonia, Parkinson's disease, and other disorders.
In people with torsion dystonia, an altered gene causes a protein called torsinA to be formed in an abnormal way. Researchers have long wondered how the abnormal version of the protein leads to the disease. The new study shows that the normal protein helps to prevent abnormally folded proteins from clumping together, or aggregating, inside the body's cells. Abnormal clumps of proteins have been identified in Parkinson's, Alzheimer's, Huntington's, and a variety of other neurological diseases. The study appears in the February 1, 2003, issue of Human Molecular Genetics. 1
Dystonias are a group of movement disorders in which sustained muscle contractions cause twisting and repetitive movements or abnormal postures. Torsion dystonia is the most severe early-onset form of dystonia. Symptoms usually begin around age 12. While the disorder typically begins in just one part of the body, it eventually spreads to other regions and can leave individuals severely disabled and confined to a wheelchair.
Much of the way proteins work depends on their shape. However, proteins are complex molecules and they often fold into abnormal shapes. Abnormally folded proteins tend to clump together in ways that may be harmful to the cell. One misfolded protein can also cause others to become misfolded.
"It's like clogging a drain," says lead investigator Guy Caldwell, Ph.D., of The University of Alabama at Tuscaloosa. "All it takes is a couple of hairs to attract others, and soon the drain can't function correctly." The protein clumps may prevent cells from carrying out normal activities. They also may make the cells less resistant to stress-related damage, he adds. Scientists have identified several proteins called chaperones that help to prevent other proteins from folding incorrectly and clumping together.
In the new study, Dr. Caldwell and his colleagues created special strains of a transparent, microscopic worm called C. elegans. These worms contained a version of green fluorescent protein (GFP) that was genetically engineered to clump together. GFP was originally isolated from jellyfish and is commonly used in biological research because it is fluorescent and easy to see in living animals. In addition to the GFP, some worms contained the normal form of torsin, while others contained an altered form and a third strain contained two types of torsin proteins.
Worms that had the normal form of torsin had many fewer fluorescent clumps than worms without this protein, and the clumps that did form were smaller and more uniform in size than those in worms with GFP alone, the researchers found. The fluorescent protein was more evenly distributed throughout the cells of the worms with normal torsin. In contrast, worms with an altered form of torsin, similar to that found in dystonia, had many separate clumps of fluorescent protein. The torsin tended to accumulate near the fluorescent clumps in the worms' cells. These findings showed that torsin helps to prevent or reduce clumping of the misfolded proteins.
The researchers also found that worms with two kinds of torsin protein had smaller protein clumps than worms with a single kind of torsin. This suggests that different kinds of torsin may sometimes work together to block formation of misfolded proteins.
This study provides an important clue about how torsion dystonia may develop, Dr. Caldwell says. Torsin is the only known chaperone-like protein to cause a human disease when it is defective. While abnormal protein clumping has never been identified in this disease, less obvious problems with protein misfolding may lead to the disease's symptoms, he adds. Such subtle effects may explain why only about 30 to 40 percent of people with the altered torsinA gene develop dystonia. People with the abnormal gene may get the disease only if stress or some other factor overrides their cells' ability to cope, Dr. Caldwell suggests. Physical stress has been linked to some forms of dystonia.
Other researchers have found torsinA in Lewy bodies - clumps of a protein called alpha-synuclein that are common in Parkinson's disease and another disorder called Lewy body dementia. Research also has shown that increasing the amount of torsinA and other suspected chaperones helps to prevent clumping of alpha-synuclein. These findings suggest that torsinA may be part of the cell's mechanism to protect against harmful effects of these clumps, Dr. Caldwell says.
Dr. Caldwell and his colleagues now plan to use their worm model to study the effects of torsins on protein aggregation during aging. Since the tiny worms age relatively quickly - with an average lifespan of 17 days - they are useful for studies of the aging process. Dr. Caldwell also has a grant from the National Institute of Neurological Disorders and Stroke (NINDS) to screen for genes that may affect torsinA's activity. These genes might be potential targets for new therapies, and they might play a role in the development of other kinds of dystonia, he says. Researchers also hope to identify compounds that enhance or supplement torsinA's ability to reduce protein clumping. This work could lead to new treatments for disorders associated with protein aggregation.
The NINDS is a component of the National Institutes of Health in Bethesda, Maryland, part of the U.S. Department of Health and Human Services, and is the nation's primary supporter of biomedical research on the brain and nervous system.
Reference:
1 Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA. "Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins." Human Molecular Genetics, February 1, 2003, Vol. 12, No. 3, pp. 307-319.- By Natalie Frazin
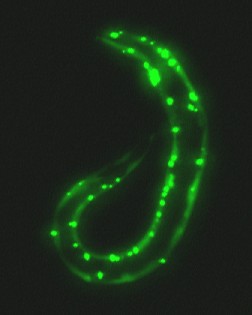
Photo caption:
A worm (C. elegans) containing green fluorescent protein clumps in the presence of normal human torsinA protein. Partial suppression of the fluorescent protein clumping can be seen. Photo credit: Guy A. Caldwell, The University of Alabama at Tuscaloosa.
Date Last Modified: Thursday, December 04, 2008




