
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-480 - Lauric Acid Diethanolamine Condensate
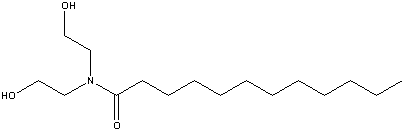
| Chemical Formula: C16H33NO3 | - | 3D Structure* |
|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | ||
Lauric acid diethanolamine condensate is widely used in cosmetics, shampoos, soaps, and related consumer products, to which there is extensive human exposure. Because of the lack of information about potential risks associated with long-term exposure, lauric acid diethanolamine condensate, coconut oil acid diethanolamine condensate, and oleic acid diethanolamine condensate were selected as representative of the class of diethanolamides for evaluation of prechronic toxicity and carcinogenic potential. Male and female F344/N rats and B6C3F1 mice were exposed to lauric acid diethanolamine condensate dermally for 14 weeks or 2 years. Genetic toxicology studies were conducted in Salmonella typhimurium, L5178Y mouse lymphoma cells, cultured Chinese hamster ovary cells, and mouse peripheral blood erythrocytes.
Groups of 10 male and 10 female rats were admin istered 0, 25, 50, 100, 200, or 400 mg lauric acid diethanolamine condensate/kg body weight in ethanol by dermal application for 14 weeks. All animals survived until study termination. Final mean body weights and body weight gains of males receiving 200 or 400 mg/kg were significantly less than those of the vehicle control group. Irritation of the skin at the site of application was observed in males receiving 100 mg/kg or greater and in females receiving 200 or 400 mg/kg. Kidney weights of females administered 200 or 400 mg/kg were significantly greater than those of the vehicle control group. There were dose-dependent increases in the incidences of nonneoplastic lesions of the skin at the site of application, including epidermal and sebaceous gland hyperplasia, chronic inflammation, parakeratosis, and ulcer.
Groups of 10 male and 10 female mice were admin istered 0, 50, 100, 200, 400, or 800 mg lauric acid diethanolamine condensate/kg body weight in ethanol by dermal application for 14 weeks. All animals survived until the end of the study, and final mean body weights and body weight gains of dosed mice were generally similar to those of the vehicle control groups. Irritation of the skin at the site of application was observed in all males and females administered 400 or 800 mg/kg. The kidney weights of males receiving 100, 400, or 800 mg/kg and females receiving 800 mg/kg were significantly greater than those of the vehicle controls. Liver weights of females administered 200 mg/kg or greater were significantly greater than those of vehicle controls. Increased incidences of nonneoplastic lesions of the skin at the site of application, including epidermal and sebaceous gland hyperplasia, chronic inflammation, parakeratosis, and ulcer, were observed in males and females receiving 200 mg/kg or greater.
Groups of 50 male and 50 female rats were admin istered 0, 50, or 100 mg lauric acid diethanolamine condensate/kg body weight in ethanol by dermal application for 104 or 105 weeks.
Survival and Body Weights
There were no significant differences between vehicle control and dosed males or females in survival or mean body weights.
Pathology Findings
There were no chemical-related differences in neoplasm incidences. Dose-related increases occurred in the incidences of nonneoplastic lesions of the skin at the site of application, including epidermal and sebaceous gland hyperplasia, hyperkeratosis, chronic inflammation, parakeratosis, and ulcer.
Groups of 50 male and 50 female mice were admin istered 0, 100, or 200 mg lauric acid diethanolamine condensate/kg body weight in ethanol by dermal application for 105 or 106 weeks.
Survival and Body Weights
There were no significant differences in survival between vehicle control and dosed males or females. Mean body weights of females that received 200 mg/kg were less than those of the vehicle controls beginning at week 33.
Pathology Findings
The incidences of hepatocellular adenoma or carcinoma (combined) were significantly increased in dosed females compared to the vehicle controls, as was the incidence of hepatocellular adenoma in the 100 mg/kg female group. There were dose-related increases in the incidences of nonneoplastic lesions of the skin at the site of application, including epidermal and sebaceous gland hyperplasia, hyperkeratosis, chronic inflammation, and parakeratosis. Dosed males had greater incidences of thyroid gland follicular cell focal hyperplasia than did the vehicle controls.
Lauric acid diethanolamine condensate was not mutagenic in Salmonella typhimurium strain TA97, TA98, TA100, or TA1535, with or without S9 metabolic activation enzymes. No increase in the frequency of mutant colonies of L5178Y mouse lymphoma cells was noted after exposure to lauric acid diethanolamine condensate, with or without S9. In cytogenetic tests with cultured Chinese hamster ovary cells, lauric acid diethanolamine condensate was shown to induce sister chromatid exchanges, but not chromosomal aberrations, with and without S9. In vivo, no increase in the frequency of micro nucleated normochromatic erythrocytes was observed in peripheral blood samples from male and female mice treated dermally with lauric acid diethanolamine condensate for 14 weeks.
Under the conditions of these 2-year dermal studies, there was no evidence of carcinogenic activity of lauric acid diethanolamine condensate in male or female F344/N rats administered 50 or 100 mg/kg or in male B6C3F1 mice administered 100 or 200 mg/kg. There was some evidence of carcinogenic activity in female B6C3F1 mice based on increased incidences of hepatocellular neoplasms. These increases were associated with free diethanolamine, which was present as a contaminant of lauric acid diethanolamine condensate.
Dermal administration of lauric acid diethanolamine condensate to rats and mice for 2 years resulted in increased incidences of epidermal and sebaceous gland hyperplasia, hyperkeratosis, chronic inflammation, and parakeratosis at the site of application.
Lauric acid diethanolamine condensate administration also resulted in increased incidences of thyroid gland follicular cell hyperplasia in dosed male mice.
Synonyms: N,N-bis(2-hydroxyethyl) dodecanamide; N,N-bis(hydroxyethyl) lauramide; N,N-bis(b-hydroxyethyl) lauramide; bis(2-hydroxyethyl) lauramide; coco diethanolamide; coconut oil amide of diethanolamine; diethanollauramide; N,N-diethanollauramide; N,N-diethanollauric acid amide; lauramide DEA; lauric diethanolamide; lauroyl diethanolamide; lauryl diethanolamide; LDA; LDE
Trade names: Clindrol 200 L; Ninol AA62; Onyxol 345; Rewomid DLMS; Rewomid
DL 203/S; Richamide 6310; Rolamid CD; Standamidd LD; Steinamid
DL 203 S; Super amide L-9A; Super amide L-9C; Synotol L-60; Unamide
J-56; Varamid ML 1
| Male
F344/N Rats | Female
F344/N Rats | Male
B6C3F1 Mice | Female
B6C3F1 Mice | |
|---|---|---|---|---|
| Doses in ethanol by dermal application | 0, 50, or 100 mg/kg | 0, 50, or 100 mg/kg | 0, 100, or 200 mg/kg | 0, 100, or 200 mg/kg |
| Body weights | Dosed groups similar to vehicle control group | Dosed groups similar to vehicle control group | Dosed groups similar to vehicle control group | 200 mg/kg group less than vehicle control group |
| Survival rates | 12/50, 18/50, 16/50 | 28/50, 26/50, 22/50 | 40/50, 37/50, 41/50 | 37/50, 40/49, 29/50 |
| Nonneoplastic effects | Skin (site of application): epidermal hyperplasia (0/50, 29/50, 44/50); sebaceous gland hyperplasia (0/50, 27/50, 44/50); hyperkeratosis (0/50, 23/50, 25/50); chronic inflammation (0/50, 10/50, 28/50); parakeratosis (0/50, 21/50, 33/50); ulcer (0/50, 4/50, 25/50) | Skin (site of application): epidermal hyperplasia (5/50, 33/50, 44/50); sebaceous gland hyperplasia (3/50, 41/50, 45/50); hyperkeratosis (0/50, 20/50, 29 50); chronic inflammation (3/50, 35/50, 34/50); parakeratosis (2/50, 12/50, 25/50); ulcer (0/50, 4/50, 25/50) | Skin (site of application): epidermal hyperplasia (4/50, 45/50, 50/50); sebaceous gland hyperplasia (1/50 45/50, 48/50); hyperkeratosis (3/50, 45/50, 49/50); chronic inflammation (1/50, 13/50, 28/50); parakeratosis (0/50, 1/50, 5/50)
Thyroid gland: follicular cell hyperplasia (18/50, 24/50, 36/50) | Skin (site of application): epidermal hyperplasia (0/50, 42/49, 50/50); sebaceous gland hyperplasia (0/50, 43/49, 45/50); hyperkeratosis (4/50, 41/49, 48/50); chronic inflammation (0/50, 24/49, 40/50); parakeratosis (0/50, 3/49, 9/50) |
| Neoplastic effects | None | None | None | Liver: hepatocellular adenoma (23/50, 32/49, 29/50); hepatocellular adenoma or carcinoma (combined) (28/50, 40/49, 36/50) |
| Level of evidence of carcinogenic activity | No evidence | No evidence | No evidence | Some evidence |
| Genetic toxicology | ||||
|---|---|---|---|---|
| Salmonella typhimurium gene mutations: | Negative in strains TA97, TA98, TA100, and TA1535 with and without S9 | |||
| Mouse lymphoma gene mutations: | Negative with and without S9 | |||
| Sister chromatid exchanges Cultured Chinese hamster ovary cells in vitro: |
Positive with and without S9 | |||
| Chromosomal aberrations Cultured Chinese hamster ovary cells in vitro: |
Negative with and without S9 | |||
| Micronucleated erythrocytes Mouse peripheral blood in vivo: |
Negative | |||
Report Date: July 1999
Pathology Tables, Survival and Growth Curves from NTP 2-year Studies
Target Organs and Incidences for 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 11, 2007
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.