 |
|
 |

Studies Affirm Tamoxifen's Long-Term Preventive Benefit
Long-term follow-up data from two cancer prevention trials conducted in the United Kingdom have confirmed that women at high risk for breast cancer continue to receive a risk-reduction benefit from tamoxifen 1 years after they have stopped taking it. That risk reduction, the reports show, is matched by another favorable development: a significantly lessened risk of serious adverse effects, such as blood clots and endometrial cancer.
The new studies also support the follow-up data 2 from a similar study conducted in the United States, the Breast Cancer Prevention Trial (BCPT), which found a continued benefit from tamoxifen after its use had ended and a stronger benefit-to-risk ratio in premenopausal women. Read more 3 


Guest Update by Dr. Joseph F. Fraumeni, Jr.
 NCI's Epidemiologic Research on Benzene Contributes to New EPA Rule NCI's Epidemiologic Research on Benzene Contributes to New EPA Rule
Since the British surgeon Percivall Pott reported in 1775 on the high frequency of scrotal cancer among chimney sweeps, studies of occupational groups have been instrumental in the discovery of environmental carcinogens and the development of preventive measures. In modern times, regulatory agencies have depended to a considerable extent on epidemiologic studies in the workplace in formulating public health policies to control hazardous exposures among both workers and the general population.
Most recently, a series of studies in NCI's Division of Cancer Epidemiology and Genetics 4 (DCEG) was considered by the U.S. Environmental Protection Agency (EPA) in establishing a new rule to limit the benzene content in gasoline and adopt controls on passenger vehicles and portable fuel containers in order to significantly reduce emissions of hazardous air pollutants. The rule was signed and submitted to the Federal Register 5 on February 9. Read more 6 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Studies Affirm Tamoxifen's Long-Term Preventive Benefit
Long-term follow-up data from two cancer prevention trials conducted in the United Kingdom have confirmed that women at high risk for breast cancer continue to receive a risk-reduction benefit from tamoxifen 1 years after they have stopped taking it. That risk reduction, the reports show, is matched by another favorable development: a significantly lessened risk of serious adverse effects, such as blood clots and endometrial cancer.
The new studies also support the follow-up data 2 from a similar study conducted in the United States, the Breast Cancer Prevention Trial (BCPT), which found a continued benefit from tamoxifen after its use had ended and a stronger benefit-to-risk ratio in premenopausal women.
"We now have additional confirmation of what we've been saying - that tamoxifen's beneficial effects don't end with the last pill, and that the risk of life-threatening side effects decreases very rapidly after your last pill," says Dr. Leslie Ford, associate director for clinical research in NCI's Division of Cancer Prevention 7. "That's what you want in a prevention agent."
Both studies, published in the February 21 Journal of the National Cancer Institute (JNCI), included follow-up data from randomized trials comparing 20 mg daily of tamoxifen versus placebo. In the IBIS-I trial, more than 7,100 participants took tamoxifen or a placebo for 5 years, and the research team provided data after a median of 8 years follow-up. In the other study, conducted entirely at the Royal Marsden Hospital in London, approximately 2,500 participants took tamoxifen or a placebo daily for 8 years, and the research team provided data after a median of 13 years follow-up.
The updated IBIS-I trial results revealed a 34-percent reduction in the risk of estrogen receptor (ER)-positive invasive breast cancer, similar to the 31-percent reduction seen when the trial results were initially reported in 2002. The updated Royal Marsden data showed a 39-percent reduction in ER-positive breast cancer, after finding no statistically significant decrease in risk when the study's results were initially reported in 1998.
In an accompanying editorial in JNCI, Dr. Umberto Veronesi and colleagues from the European Institute of Oncology in Milan wrote that the study results "suggest a true preventive effect and not merely transient risk reduction" and "highlight [tamoxifen's] favorable risk-benefit ratio in younger women, among whom severe toxicity is rare."
In both trials, Dr. Ford notes, participants were allowed to be on hormone replacement therapy (HRT), which could mitigate tamoxifen's impact. In the 13,000-participant BCPT, which saw a greater risk reduction - 49 percent when preliminary results were reported and 43 percent on long-term follow-up - HRT was not allowed.
The reluctance among U.S. primary care physicians to prescribe tamoxifen as a preventive agent has been well documented, with much of it attributed to their lack of familiarity and comfort with the drug.
"There are individual patients who…may not choose tamoxifen because of appropriate concerns about toxicity," says Dr. Lawrence Wickerham, associate chairman of the National Surgical Adjuvant Breast and Bowel Project (NSABP), which oversaw BCPT and another breast cancer prevention trial, STAR 8. "But a substantial barrier to greater tamoxifen use has been clinicians. These results may help to eliminate that."
As was anticipated, the risk-reduction benefit in both trials was limited to ER-positive breast cancer. Tamoxifen works by blocking cells' estrogen receptors, preventing estrogen from binding to potentially mutated cells and fueling their proliferation, so it was not expected to help prevent non-ER-expressing cancers.
There are no proven methods to specifically target or predict which high-risk women are likely to develop ER-positive breast cancer. But, explains Dr. Wickerham, 60 to 70 percent of breast cancers are ER positive, so using tamoxifen as a prevention agent is already a fairly targeted pursuit.
In addition, the Gail model that is frequently used to identify women at high risk for invasive breast cancer, Dr. Ford says, "contains many risk factors of ER-positive disease, such as age at menarche, age at first birth, and age at menopause." All of these are indicators of long-term exposure to estrogen.
By Carmen Phillips
|
 |
 |

Guest Update by Dr. Joseph F. Fraumeni, Jr.
NCI's Epidemiologic Research on Benzene Contributes to New EPA Rule
 Since the British surgeon Percivall Pott reported in 1775 on the high frequency of scrotal cancer among chimney sweeps, studies of occupational groups have been instrumental in the discovery of environmental carcinogens and the development of preventive measures. In modern times, regulatory agencies have depended to a considerable extent on epidemiologic studies in the workplace in formulating public health policies to control hazardous exposures among both workers and the general population. Since the British surgeon Percivall Pott reported in 1775 on the high frequency of scrotal cancer among chimney sweeps, studies of occupational groups have been instrumental in the discovery of environmental carcinogens and the development of preventive measures. In modern times, regulatory agencies have depended to a considerable extent on epidemiologic studies in the workplace in formulating public health policies to control hazardous exposures among both workers and the general population.
Most recently, a series of studies in NCI's Division of Cancer Epidemiology and Genetics 4 (DCEG) was considered by the U.S. Environmental Protection Agency (EPA) in establishing a new rule to limit the benzene content in gasoline and adopt controls on passenger vehicles and portable fuel containers in order to significantly reduce emissions of hazardous air pollutants. The rule was signed and submitted to the Federal Register 5 on February 9.
In developing this policy, EPA was influenced by the results of a 20-year collaborative research program 9 in which DCEG investigators worked closely with scientists at the Chinese Center for Disease Control and Prevention (or CDC, formerly known as the Chinese Academy of Preventive Medicine). The goal of this study was to evaluate the cancer risks in a large cohort of Chinese workers heavily exposed to benzene, a known carcinogen that is widely prevalent in the environment. Using detailed exposure assessment data, the cohort study, published in the Journal of the National Cancer Institute (1997) 10, was able to uncover an increased risk of acute nonlymphocytic leukemia and myelodysplastic syndrome at exposures below 10 parts per million (ppm). There was also evidence linking benzene to other forms of leukemia and possibly non-Hodgkin lymphoma.
These findings were followed by molecular epidemiology studies utilizing a panel of biomarkers to help identify the underlying mechanisms of benzene-related leukemia, including precursor conditions and susceptibility states. It was reported in the American Journal of Industrial Medicine (1996) 11 that hematologic toxicity occurred at lower levels of benzene exposure than generally appreciated. This prompted a larger study published in Science (2004) 12 that uncovered hematotoxicity among workers exposed to levels under 1 ppm in the air, the current U.S. occupational standard. In addition, variants in several genes were associated with a greater decrease in white blood cell counts among exposed workers.
While this long-term collaborative U.S.-China project focused on the hazards of benzene in the workplace, it is known that large segments of the population around the world are exposed to small amounts of benzene on a daily basis, especially through inhaled air that contains benzene from vehicular and other sources. Thus, the quantitative dose-related health effects from workplace exposures have contributed not only to lowering the benzene occupational standard in China, but also to guiding the risk assessment process leading to controls on environmental benzene exposure in the United States.
In addition to several intramural investigators at NCI, this binational collaboration involved a team of Chinese investigators led by Drs. Songnian Yin and Guilan Li from the Chinese CDC. The molecular epidemiology components also included Drs. Martyn Smith, Luoping Zhang, and Stephen Rappaport, at the University of California, Berkeley.
|
 |
 |
Male Tobacco Switchers Have Increased Mortality Rates
Men who switch from smoking cigarettes to smokeless tobacco (chewing tobacco and snuff) had a higher rate of death from all causes, lung cancer, coronary heart disease, and stroke than former cigarette smokers who quit using tobacco entirely, according to study results published online February 15 in Tobacco Control.
Dr. Michael J. Thun of the American Cancer Society (ACS) and colleagues identified 4,443 men who were switchers and 111,952 men who quit tobacco entirely from the Cancer Prevention Study II. This ongoing prospective study includes 1.2 million U.S. adults; it began in the fall of 1982 with a follow-up 20 years later. In the study, ACS volunteers asked friends, neighbors, and acquaintances who were 30 and older to complete a confidential, four-page mailed questionnaire on their smoking habits, alcohol intake, education, and other demographic characteristics.
Most switchers in the study began using smokeless tobacco within a year of quitting smoking and had used smokeless tobacco for an average of 9 years. The researchers found that switchers had significantly higher rates of death from all causes, lung cancer, coronary heart disease, and stroke than men who had never used tobacco or were former cigarette smokers and quit using tobacco entirely. Additionally, researchers found differences in demographic characteristics and lifestyle. Switchers tended to be less educated, more often employed in blue-collar occupations, more likely to engage in heavy exercise (possibly associated with a blue-collar occupation), and reported consuming fewer fruits, vegetables, and alcohol, but more dietary fat than those who quit using tobacco entirely. The analyses adjusted for other risk factors and the number of years the switchers smoked, number of cigarettes smoked per day, and age at quitting smoking.
The authors noted that "Our results support the stand that smokers who want to quit should be offered safe, clinically proven treatments for smoking cessation, including pharmacotherapies such as medicinal nicotine or antidepressants, behavioral counseling, and telephone quitlines."
Researchers Discover Biomarkers for Diagnosing Liver Cancer
A team of researchers led by Dr. Xin Wei Wang of NCI's Center for Cancer Research 13 (CCR) has identified a five-gene signature that can be used to diagnose early-stage hepatocellular carcinoma (HCC). The findings appeared online February 20 in Clinical Cancer Research.
Currently, an elevated level of the protein alpha-fetoprotein (AFP) in the serum is the only known diagnostic biomarker for HCC. This elevated level of AFP is detectable in only about one-third of patients with early-stage HCC. Consequently, most HCC is not diagnosed in its early stages, leading to high mortality from the disease.
Using microarray techniques to examine a range of gene expression profiles from 218 HCC specimens, Dr. Wang's team and collaborators at the Liver Cancer Institute of Fudan University in China identified five candidate genes that were overexpressed in HCC. This finding, in combination with Dr. Wang's earlier research into the tumor microenvironment 14, suggests a potential means for increased accuracy in diagnosis and monitoring recurrence during treatment.
Men with Low-Risk Prostate Cancer Often Choose Treatment over Surveillance
Men diagnosed with prostate cancer who have a very low risk of dying of the disease and are eligible for active surveillance - also known as watchful waiting - rarely choose this option over treatment, researchers reported last week at the Prostate Cancer Symposium in Orlando, FL. An analysis of data from the CaPSURE prostate cancer registry found that only 9 percent of those eligible for surveillance selected the option.
Active surveillance involves the careful monitoring of the disease until such time as treatment is needed. Physicians may recommend active surveillance for older patients with early-stage disease 15 who are more likely to die of another cause; some forms of prostate cancer progress so slowly that they never cause any harm.
In the study, older men chose surveillance more often than younger men. Men over age 70 were 26 times more likely to choose surveillance than men younger than 63, while men between 63 and 70 were 5 times more likely to choose surveillance than those younger than 63.
The researchers analyzed data on 1,886 patients diagnosed between 1999 and 2004. Of this group, 310 had very low-risk disease based on 5 criteria including prostate-specific antigen level, Gleason score, and stage. Only 28 of the 310 candidates (9 percent) chose surveillance.
Anxiety caused by a diagnosis of cancer may be a factor driving men to choose treatment, said Dr. Daniel Barocas of the New York-Presbyterian Hospital-Weill Cornell Medical College, who presented the findings. Previous studies have estimated that about one in five men who choose surveillance will go on to develop prostate cancer that cannot be treated.
Dr. Eric Klein of the Cleveland Clinic, who commented on the findings, said that an issue for clinicians is the lack of adequate tools to identify which patients are likely to need future treatment and which are not. A second issue is the lack of clinical tools to determine when a person who has chosen surveillance needs treatment.
"Until we have those tools there is likely to be some hesitancy to choose surveillance," said Dr. Klein.
High Doses of Vitamin D Hormone Boost Prostate Cancer Therapy
Some men with advanced prostate cancer may benefit from taking the chemotherapy drug docetaxel 16 in combination with a pill that delivers high doses of the vitamin D hormone, a randomized phase II clinical trial has found. The pill, DN-101, contains a biologically active form of vitamin D called calcitriol. Preclinical studies have suggested that high amounts of calcitriol may benefit patients with prostate cancer.
In the trial, the addition of calcitriol to docetaxel did not lead to an increase in toxic side effects, and it was associated with a reduced risk of death (by approximately a third over docetaxel alone). DN-101 is thought to work by producing much higher blood levels of calcitriol than the body can produce from dietary vitamin D or vitamin D supplements.
Dr. Tomasz Beer of the Oregon Health & Science University Cancer Institute and his colleagues reported the findings in the February 20 Journal of Clinical Oncology. Dr. Beer's team first tested calcitriol plus docetaxel in a small group of patients several years ago.
The new results are from the Androgen Independent Prostate Cancer (AIPC) Study of Calcitriol Enhancing Taxotere (ASCENT 17), a randomized, double-blinded, placebo-controlled trial. It included 250 men at 48 sites in the United States and Canada. The men had advanced prostate cancer that no longer responded to hormonal therapy, a condition called androgen-independent prostate cancer.
"The purpose of this study was to see if there was sufficient evidence for undertaking a phase III trial, and the survival data clearly demonstrated this," said Dr. Beer. A final-stage clinical trial 18 is underway to test the effects of docetaxel plus calcitriol on overall survival in a larger group of patients.
Secondary Sarcomas Threaten Childhood Cancer Survivors
Previous research has shown that children who received radiation therapy for cancer treatment and survived at least 5 years face an increased risk years later of developing bone and soft tissue sarcomas. Now, new findings reported in the February 21 JNCI reveal that - regardless of what treatments they received - childhood cancer survivors are nine times more likely to develop sarcomas than are their age-matched peers in the general population. On average, the secondary sarcoma appears 11 years after the initial cancer diagnosis.
When researchers compared the 104 patients who developed secondary sarcomas with the 14,258 other survivors from the Childhood Cancer Survivors Study 19 (CCSS) who did not, a number of additional risk factors emerged. Children originally given radiation therapy had 3.1 times the risk. Three other risk factors more than doubled the risk of secondary sarcoma: a history of other secondary neoplasms and high doses of chemotherapy with either anthracyclines or with alkylating agents. Children with an original primary diagnosis of sarcoma were at greatest risk, however, with a 10.1 times higher risk than children who survived other types of cancer.
"Diagnosis of a sarcoma can sometimes be elusive because symptoms are often nonspecific," wrote lead author Dr. Tara O. Henderson of the University of Chicago. She urged clinicians and researchers to take these new findings into consideration as they evaluate the risk for second cancers in this highly vulnerable population.
This is 1 of more than 60 studies based on CCSS, the largest database of prospectively gathered information on childhood cancer survivors ever compiled. The study 20 is supported by NCI and involves 27 participating pediatric oncology research centers. Study data are available to other investigators in the research community.
|
 |
 |
A Conversation with...Dr. Walter Willett
 Dr. Walter C. Willett is the Fredrick John Stare Professor of Epidemiology and Nutrition at Harvard School of Public Health and a founding investigator of the Nurses’ Health Study II 21. He spoke about diet and cancer at NCI on February 22-23 as a Division of Cancer Epidemiology and Genetics 4 Visiting Scholar. Dr. Walter C. Willett is the Fredrick John Stare Professor of Epidemiology and Nutrition at Harvard School of Public Health and a founding investigator of the Nurses’ Health Study II 21. He spoke about diet and cancer at NCI on February 22-23 as a Division of Cancer Epidemiology and Genetics 4 Visiting Scholar.
What have we learned from the completed long-term studies of diet and cancer risk?
What has become clear in the last 10 or so years is that by far the most important impact of diet on cancer is mediated through body weight, resulting from overweight and inactivity. I think it's important to keep in mind that it's not just being lean that's beneficial, it's also being physically active - staying lean and active is the most important thing one can do to prevent cancer, after not smoking.
There are still many specific components of diet that do seem to be important for cancer prevention, such as keeping red meat consumption, particularly processed meat, relatively low. The total percentage of calories in the diet from fat doesn't seem to be important. There may still be some modest benefit of higher fruit and vegetable intake for cancer prevention, but it's not the 'big bang' it was thought to be 15 or 20 years ago.
But it's also important that we don't adopt a lifestyle just for preventing one disease, and we particularly have to pay attention to cardiovascular disease, as that is still the number one cause of death. But in general, many of the same things we do for cardiovascular disease are going to have an impact on cancer.
During your visit, you mentioned that prospective observational studies might be
more appropriate than randomized trials for resolving questions about diet and cancer
prevention. What are the difficulties in doing randomized trials of dietary interventions?
The paradigm of randomized trials came from clinical medicine, where you have a sick patient and you know what the right time to start the intervention is - it's when they get sick. For prevention, particularly for prevention by lifestyle factors, the questions don't always fit the randomized trial paradigm, partly because very often we don't know when to start the intervention, and partly because there's a lot of evidence that, for many of these factors, the relevant period is early in life. It's impossible to imagine doing trials where you start in adolescence and follow people for 40 years.
Also, we're often looking at factors that are much more complicated to change than just taking a pill or placebo - to actually change human behavior is difficult. It's not impossible, but to keep the two trial groups separated is the real challenge. That can be a serious problem in a randomized trial, particularly when you're dealing with items that are on the shelves of your grocery store, in pill form or in food form.
What areas of research in the field of diet and cancer are investigators really excited about right now?
I think there are many different areas to investigate where there's promise, but the evidence is not clear, yet. Vitamin D is clearly a potentially important topic, where there are many suggestions that there will be important effects on cancer. Among the other aspects of diet, I think there may be a number of specific foods or nutrients that have modest beneficial effects, and when you 'package' them all together, there may be a very substantial effect.
Another critical area is milk consumption and calcium intake, because there are now quite a few studies showing that higher intakes of calcium or dairy products are related to increased risk of fatal prostate cancer. And that's important, because the national recommendation to drink three glasses of milk a day would double dairy consumption and production in the United States. As we have no good evidence suggesting it would reduce fractures and quite a few studies showing a relationship with fatal prostate cancer, that's an important area to resolve.
Another broad area to explore is the effect of diet during earlier periods in life. There is much indirect evidence that diet is likely to have an important effect during that period of life. |
 |
 |

Zoledronate to Preserve Bone Mineral Density
Name of the Trial
Phase II Randomized Study of the Effect of Zoledronate Versus Observation on Bone Mineral Density of the Lumbar Spine in Patients Undergoing Risk-Reducing Excision of Both Ovaries (GOG-0215). See the protocol summary at http://cancer.gov/clinicaltrials/GOG-0215.
 Principal Investigators Principal Investigators
Dr. David Alberts, Dr. Larissa Korde, Dr. Gus Rodriguez, and Lisa Hess, Gynecologic Oncology Group
Why This Trial Is Important
Women whose family history or genetic make-up put them at high risk of ovarian cancer may choose to have their ovaries removed as a preventive measure. This surgery, however, can lead to early and accelerated bone loss in premenopausal women. The ovaries produce estrogen, a hormone that normally helps prevent bone loss.
Drugs called bisphosphonates have been shown to reduce the bone loss caused by menopause and some other medical conditions. Now doctors want to know if a bisphosphonate called zoledronate 22 (Zometa) can help prevent bone loss in premenopausal women undergoing risk-reducing surgery to remove both ovaries.
In this trial, all women will have their bone mineral density checked prior to surgery and receive calcium and vitamin D supplements for 18 months following surgery. In addition, half of the women will be randomly assigned to receive intravenous zoledronate once every 6 months. The remaining women will be monitored without receiving additional treatment. Bone mineral density will be checked at 9 months and 18 months following surgery.
"Premenopausal women undergoing risk-reducing surgery typically are not monitored adequately for osteoporosis, but it is a very serious side effect for these women," said Dr. Alberts. "All women in this trial will benefit from very close observation, and those receiving zoledronate will be getting a drug with a record of preventing and reversing bone loss in cancer patients."
Who Can Join This Trial
Researchers will enroll 222 premenopausal women at increased risk of ovarian cancer who are undergoing surgery to remove both ovaries. See the list of eligibility criteria at http://cancer.gov/clinicaltrials/GOG-0215.
Study Sites and Contact Information
Study sites in the United States are recruiting patients for this trial. See the list of study contacts at http://cancer.gov/clinicaltrials/GOG-0215 or call NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237) for more information. The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |
 Blumberg to Receive National Public Service Award Blumberg to Receive National Public Service Award
Dr. Peter Blumberg, a senior investigator in the Molecular Mechanisms of Tumor Promotion section of CCR's Laboratory of Cancer Biology and Genetics, was selected to receive a National Public Service Award for his work with deaf scientists and his contributions to cancer research. The National Public Service Awards are presented jointly by the American Society for Public Administration and the National Academy of Public Administration to recognize outstanding practitioners who have spent most of their careers in public service. The award will be presented at a luncheon during the 2007 American Society for Public Administration National Conference on March 26 in Washington, DC.
"Understanding NCI" Teleconference Slated for March 7
The next "Understanding NCI" teleconference will take place on March 7 from 1:00 to 2:00 p.m., EST. The topic is "How the Patient Navigator Program Helps Cancer Patients." The call will feature Dr. Roland Garcia, program director of the Patient Navigator Research Program in NCI's Center to Reduce Cancer Health Disparities, and Dr. Beth Calhoun, co-principal investigator of the Patient Navigator Research Program in Chicago.
No registration is required and participation is free. Within the U.S., the teleconference can be accessed toll free at 800-857-6584; the passcode is PNP. Toll-free playback will be available through April 7 at 800-873-2035.
For additional information, contact the Office of Liaison Activities 23 at 301-594-3194 or liaison@od.nci.nih.gov. Information on the Patient Navigator Program can be found at http://CRCHD.cancer.gov.
Breast Cancer Conference Scheduled for March
On March 26 and 27, the meeting "Preoperative Therapy in Invasive Breast Cancer: Reviewing the State of the Science and Exploring New Research Directions" will take place in the Natcher Conference Center on the NIH campus. The meeting is sponsored by the Cancer Therapy Evaluation Program 24 in NCI's Division of Cancer Treatment and Diagnosis 25.
The conference will seek to determine the state of the science on clinical use of preoperative therapy in breast cancer, as well as identify future research agendas. Leading breast cancer physicians will present the state of the science and engage in a panel discussion in which audience participation is encouraged.

If Memory Serves...
The first staff at NCI was comprised of Harvard personnel, most of whom had been working in the laboratory of Dr. Joseph Schereschewsky in Cambridge, MA, which was later led by Dr. Carl Voegtlin (the first Chief of NCI) after Dr. Schereschewsky's retirement. Both Dr. Schereschewsky and Dr. Voegtlin were commissioned officers of the Public Health Service. (Read more 26)
For more information about the birth of NCI, go to http://www.
cancer.gov/aboutnci/ncia.
The target audience for this meeting includes breast cancer physicians - medical and radiation oncologists, radiologists, pathologists, surgeons, and others - as well as general interventional radiologists and surgeons. Patient advocates are welcome. There is no charge for this meeting, but preregistration is requested. The meeting will be webcast at http://videocast.nih.gov and Continuing Medical Education credit is available. Information about registration, the agenda, and faculty list are available at http://ctep.cancer.gov/bcmeeting.
Chromosome Biology Conference Slated for April
Registration is open for the NCI Symposium on Chromosome Biology, which will take place April 26-27 in the Natcher Conference Center on the NIH campus.
The meeting is sponsored by the Center of Excellence in Chromosome Biology in NCI's Center for Cancer Research. Topics to be covered include transcriptional regulation, chromatin structure, epigenetics, DNA replication and repair, and nuclear architecture.
There is no fee for this conference, but space is limited and registration is required by March 26. The deadline for abstract submission is March 16. For additional information, registration, and poster abstract submission, go to www.palladianpartners.com/cecb2007.
|
|
 |
 |

The Trials and Tested Agents of Breast Cancer Chemoprevention
Several phase III, randomized breast cancer chemoprevention trials have been completed and most have reported long-term follow-up data. Two other large trials are underway, one in England and one in Canada. The following is a brief primer on some of these trials.
Completed Trials
Royal Marsden - Randomized high-risk women to tamoxifen 1 or placebo for 8 years. Initial results showed no decrease in breast cancer risk, but recent follow-up data showed a 39-percent reduction in ER-positive invasive breast cancer.
IBIS-I - Randomized high-risk women to tamoxifen or placebo for 5 years. Initial results showed a 31-percent reduced risk of ER-positive invasive breast cancer; recent follow-up data showed a 34-percent reduction.
BCPT - The first U.S.-based breast cancer chemoprevention clinical trial. Initial results showed a 49-percent reduction in breast cancer among women on tamoxifen, with an increased risk of endometrial cancer and blood clots. Longer-term follow-up revealed a 43-percent risk reduction.
STAR - Compared tamoxifen to the anti-osteoporosis drug raloxifene. Raloxifene was as effective as tamoxifen at reducing breast cancer risk and was associated with fewer serious adverse effects. Tamoxifen was more effective at reducing the risk of noninvasive breast cancers.
RUTH - Randomized approximately 10,000 postmenopausal women to raloxifene or placebo. Raloxifene was associated with a reduced risk of invasive, primarily ER-positive breast cancer, but also increased the risk of fatal strokes and blood clots.
MORE/CORE - Randomized participants to 60 mg/day of raloxifene or placebo. Over 8 years there was a 66-percent reduction in risk of invasive breast cancer (primarily ER-positive). Raloxifene use was associated with an increased risk of blood clots.
Ongoing Trials
IBIS-II - Launched in 2004 in the United Kingdom, IBIS-II will randomize 6,000 postmenopausal, high-risk women to the aromatase inhibitor (AI) anastrozole 27 or placebo. A parallel trial is comparing anastrozole to tamoxifen in 4,000 women who have had a precancerous lesion in their breast removed.
MAP.3 - Launched last year by NCI-Canada, this trial 28 will randomize high-risk women to placebo or to the AI exemestane 29.
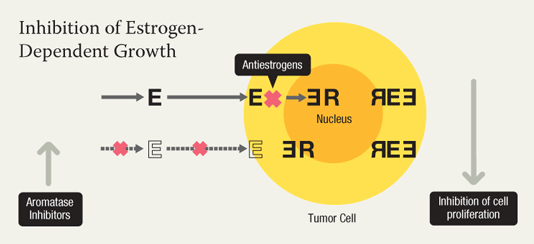 Two drugs - Two mechanisms of action
Two drugs - Two mechanisms of action
Antiestrogens such as tamoxifen bind to the estrogen receptors on breast cancer cells, preventing the hormone from binding to the cell. This keeps the cell from growing and proliferating. Aromatase inhibitors such as anastrozole and letrozole inhibit estrogen production by the body. This can slow or stop the growth of breast cancer cells that need estrogen to grow. Both types of drugs are being tested as ways to reduce breast cancer risk. |
 |
|
Table of Links
| 1 | http://www.cancer.gov/cancertopics/druginfo/tamoxifencitrate |
| 2 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_111505/page2 |
| 3 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_022707/page2 |
| 4 | http://dceg.cancer.gov |
| 5 | http://www.epa.gov/otaq/regs/toxics/msatfr.pdf |
| 6 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_022707/page3 |
| 7 | http://www.cancer.gov/prevention |
| 8 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_041806/page2 |
| 9 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_010307/page9 |
| 10 | http://dceg.cancer.gov/pdfs/00027CC4.pdf |
| 11 | http://dceg.cancer.gov/cgi-bin-pubsearch/pubsearch/index.pl?page=abstract&I
D=2654&project=dceg |
| 12 | http://dceg.cancer.gov/pdfs/lan30617742004.pdf |
| 13 | http://ccr.nci.nih.gov |
| 14 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_081506/page5 |
| 15 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_012307/page5 |
| 16 | http://www.cancer.gov/cancertopics/druginfo/docetaxel |
| 17 | http://clinicaltrials.gov/ct/show/NCT00043576?order=1 |
| 18 | http://www.cancer.gov/clinicaltrials/011-007 |
| 19 | http://www.stjude.org/epidemiology/0,2081,864_5472_21715,00.html |
| 20 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_101706/page4 |
| 21 | http://www.channing.harvard.edu/nhs |
| 22 | http://www.cancer.gov/cancertopics/druginfo/zoledronicacid |
| 23 | http://ola.cancer.gov |
| 24 | http://ctep.cancer.gov |
| 25 | http://www.cancer.gov/dctd |
| 26 | http://www.cancer.gov/aboutnci/if-memory-serves |
| 27 | http://www.cancer.gov/cancertopics/druginfo/anastrozole |
| 28 | http://www.cancer.gov/clinicaltrials/CAN-NCIC-MAP3 |
| 29 | http://www.cancer.gov/cancertopics/druginfo/exemestane |
|
 |