Justification
Authorizing Legislation: Section 301 of the Public Health Service Act, as amended. Reauthorizing legislation will be submitted.
Budget Authority
| FY 2002 Actual |
FY 2003 Amended
President's Budget |
FY 2004 Estimate |
Increase or Decrease |
| FTEs |
BA |
FTEs |
BA |
FTEs |
BA |
FTEs |
BA |
| 412 |
$890,816,000 |
414 |
$957,626,000 |
407 |
$994,411,000 |
(7) |
$36,785,000 |
Introduction
As a nation, we are in the midst of a demographic shift unprecedented in history. There are currently 35 million Americans over the age of 65 – more than at any other time in history. Of these, over 4 million are over 85, and some 65,000 have attained their hundredth birthday. In the coming years, the ranks of American elders are expected to swell; between now and 2030, the number of individuals age 65 and older likely will double, reaching 70.3 million and comprising a larger proportion of the entire population, up from 13 percent today to 20 percent in 20301. Of great interest is the explosive growth anticipated among those most at risk of disease and disability, people age 85 and older: Their ranks are expected to grow from 4.3 million in 2000 to at least 19.4 million in 2050.
The aging of the population presents a number of social and economic challenges as increasing numbers of Americans reach retirement age. It also has important implications for our nation’s health. For example, more than half of all Americans over age 65 show evidence of osteoarthritis in at least one joint2. Over half of Americans over age 50 have osteoporosis or low bone mass3. Cardiovascular disease, cancer, and diabetes remain common among older Americans. And as many as 4.5 million Americans suffer from Alzheimer’s disease (AD)4.
However, we now know that aging itself is not the cause of disease, disability, and frailty. Rather, disease and disabling processes, influenced by age-related changes in the body and by unhealthy choices and sedentary lifestyles, are the most important factors in compromising the quality of life for older people. This fundamental shift in thinking was reinforced most recently with insights from the National Long Term Care Survey (NLTCS). According to this study, the rate of disability among older Americans dramatically declined from the 1980s through the mid- 1990s, even among people age 85 and older. These findings, along with evidence from a number of clinical trials and studies, suggest more strongly than ever that disease and disability can be delayed or even prevented through specific interventions. The challenge now is to maintain and even accelerate the trend in declining disability and to reduce rates of disease amid a steep rise in the number and proportion of older people.
The mission of the NIA is to improve the health and well being of older Americans through an extensive program of high-quality research. The NIA’s portfolio is vast and emphasizes research aimed at increasing the “healthspan,”or years of healthy, active life expectancy. With guidance from the National Advisory Council on Aging, the NIA conducts and supports research on the biochemical, genetic, and physiological mechanisms of aging in humans and animal models; the structure and function of the aging nervous system; social and behavioral aspects of aging processes and the place of older people in society; and the pathophysiology, diagnosis, treatment, and prevention of age-related diseases, degenerative conditions, and disabilities. In all of its efforts, the Institute pays special attention to reducing health disparities among different groups of Americans. NIA-supported researchers can be found in all fifty states, and the Institute also conducts an active program of training opportunities for researchers wishing to become involved in aging research.
NIA and the NIH Roadmap
In September 2003, the NIH Director announced the creation of the NIH Roadmap for Medical Research, a series of initiatives to transform the nation’s medical research capabilities and speed the movement of research discoveries from the laboratory into clinical practice. A number of the NIH Roadmap initiatives are particularly relevant to aging research. For example:
- The Molecular Libraries initiative will offer biomedical researchers access to small molecules that can be used as probes to study cellular pathways in greater depth. These probes can be used to help assess the effects of an intervention (see, for example, “Mimicking Caloric Restriction to Increase Longevity in Animal Models,” page 9). Small molecule development is also crucial to the development of drugs for a variety of age-related conditions. And the development of small molecule libraries will speed the refinement of molecular imaging techniques, including those for imaging brain function, which may greatly enhance our ability to diagnose and monitor neurological conditions such as AD.
- A major component of the NIH Roadmap is Re-engineering the Clinical Research Enterprise. Clinical trials are necessary to the development of new treatments for age-related conditions, but such studies among older people are often complicated by the presence of concurrent medical conditions, or the fact that study participants may be taking several medications. One answer is the frequent use of populations established for longitudinal studies of one endpoint or condition to study an additional condition – for example, to assess AD risk factors within the context of a study of cardiovascular health. Promoting the use of common data sets and compatible databases in multiple studies, as the NIA is currently doing through its newly-established Longevity Consortium (page 14) and through the National Alzheimer’s Coordinating Center, will facilitate this.
- Clinical Research Training is a critical need in aging research. The NIA supports a variety of training programs in age-related research, including the Paul B. Beeson Career Development Awards in Aging, which provides support to junior to mid-career faculty members in strong research environments to enable them to gain skills and experience in clinical aging research and to establish an independent program of research in this field. However, additional opportunities for training are needed.
- Geriatrics researchers are recognizing an emerging emphasis on Interdisciplinary Research in aging. Studies of interactions between biology and behavior, and economics and demographics, continue to offer unique insights regarding the behavioral, social, economic, and health consequences of aging.
In this narrative, the Institute focuses on recent progress and future directions for research in four key areas: Section I) Alzheimer’s disease and the neuroscience of aging; Section II) reducing disease and disability; Section III) the biology of aging; and Section IV) the behavioral and social aspects of growing older. NIA-supported research on health disparities is described in Section V.
Alzheimer's Disease And The Neuroscience Of Aging
Alzheimer’s disease (AD) is a progressive, currently irreversible brain disorder. People with AD gradually suffer memory loss and a decline in thinking abilities, as well as major personality changes. These losses in cognitive function are accompanied by pathologic changes in the brain, including the buildup of insoluble protein deposits called amyloid plaques and the development of neurofibrillary tangles, which are abnormal collections of twisted protein threads found inside nerve cells. Such changes result in death of brain cells and breakdown of the connections between them. AD advances gradually but inexorably, from early, mild forgetfulness to a severe loss of mental function called dementia. Eventually, people with AD become dependent on others for every aspect of their care. The risk of developing AD increases exponentially with age, but it is not a part of normal aging.
AD is the most common cause of dementia among people age 65 and older and is a major public health issue for the United States because of its enormous impact on individuals, families, the health care system, and society as a whole. Scientists now estimate that as many as 4.5 million people currently suffer with the disease, and this number is expected to increase to 13.2 million persons by 2050, an almost 3-fold increase.5
This update on advances in AD and related neuroscience includes findings from across the spectrum of research, from the development of a new mouse model that will provide important insights into the etiology of the disease to clinical trials of interventions to prevent or arrest the course of the disease. We also discuss important findings in other neurological diseases, including Parkinson’s disease and amyotrophic lateral sclerosis.
The Genetics of Neurological Disease
A genetic locus for age-at-onset of Alzheimer disease and Parkinson Disease. Last year, investigators identified a region on chromosome 10 that contained a locus associated with age at onset (AAO) in both AD and Parkinson’s disease (PD). Recently, these investigators used a novel scientific approach called “genomic convergence,” which combines complementary lines of genetic evidence, and identified a gene, GSTO1, that appears to modify AAO in AD and PD. GSTO1 may be involved with inflammatory processes – of particular interest due to the possible role of inflammation in these two diseases. While further studies are needed to confirm that this is an AAO gene for AD and PD, further study of this gene and the pathway it affects may open new avenues of research for delaying onset of disease in patients at risk for these disorders.
Synuclein gene triplication as a cause of Parkinson’s Disease (PD). Previous work has shown that mutations in the gene that produces a protein called synuclein are one cause of PD, and that synuclein is a major component of Lewy bodies, the pathological hallmark of PD. However, the precise relationship between synuclein, Lewy body formation, and PD was not clear. Recently, NIH investigators screened members of a large family with the inherited form of the disease and found that affected members have three copies of the region on which a single synuclein gene is normally found. The additional copies of the synuclein gene result in overproduction of the protein, which is then deposited in the brain in the form of Lewy bodies. These findings provide strong support for the hypothesis that synuclein is at the center of the etiology and pathogenesis of Parkinson’s disease and that simple overproduction of the protein can cause its deposition in the brain. This finding will have a profound impact on our understanding of the disease mechanisms and may eventually influence treatment strategies.
Early Diagnosis of AD
Early diagnosis of AD benefits affected individuals and their families, clinicians, and researchers. For patients and their families, a definitive early diagnosis provides the opportunity to plan and to pursue options for treatment and care while the patient can still take an active role in decision-making. For clinicians, accurate early diagnosis facilitates the selection of appropriate treatments, particularly as new interventions are developed to stop or slow progression of symptoms. And for researchers, earlier and more accurate diagnosis may facilitate clinical studies of new therapies and preventive measures by allowing early intervention, before cognitive loss becomes significant.
Research suggests that the earliest AD pathology begins to develop in the brain long before clinical symptoms yield a diagnosis, and scientists are now searching for reliable, valid, and easily attainable biological markers that can identify cases very early in the course of disease. Eventually, very early diagnosis of AD may be possible through combinations of strategies to image the brain, along with genetic, biological, clinical, and neuropsychological assessments.
Brain chips dip into genes affecting aging and cognitive decline. Identifying changes in gene activity in the brain throughout life may suggest genes that are protective of brain health, as well as those that contribute to age-related brain disorders when altered; this knowledge could point to novel sites for therapeutic interventions. In a recent study, young, middle-aged, and aged rats were trained on two memory tasks. Investigators then used a powerful microarray chip technique to identify genes in the hippocampus – a brain area important for learning – whose expression changed with aging, as well as those that were involved in age-related changes in the performance of the rats on the memory tests. From this analysis, they identified 146 aging- and cognitive-related genes (ACRGs) and assigned them to categories of specific cell processes or functions. Based on the pattern of changes in the ACRGs, they posited a model of brain aging in which loss of neuronal processes and increase of myelin turnover fuel brain inflammation, eventually leading to impaired neuronal function and cognition. Most of the gene expression changes were seen in mid-life, before cognition was impaired, suggesting that such changes in early adulthood might initiate cellular or biological changes that could lead to functional changes later in life.
Visual memory predicts AD more than ten years before diagnosis. Investigators recently found that individuals who scored six or more errors on the Benton Visual Retention Test, which measures perception of spatial relations and memory for newly learned material, up to fifteen years prior to the study had approximately twice the risk of developing AD as did those with zero to five errors. The interval between the test results and the development of the disease is significantly longer than that seen in previous studies of the relationship between cognitive test performance and AD. This finding suggests that with AD, manifestation of the disease process may begin much earlier than was believed previously, and that AD may be best represented as a chronic disease that is initiated early and promoted by many factors throughout life.
Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Investigators using functional magnetic resonance imaging (fMRI) recently demonstrated that patients both with AD and with mild cognitive impairment (MCI), a population at high risk for developing AD, showed less activation in the medial temporal lobes during a memory task than elderly individuals with no cognitive impairment. The medial temporal lobes include the hippocampus and other brain structures important to the formation of memories. These results suggest that decreased activation in the medial temporal lobes during a memory encoding task as measured using fMRI may be a specific and early marker of neurodegeneration in AD, and that fMRI is sufficiently sensitive to detect changes during the early (MCI) phase of the disease.
Environmental Factors and AD
There is a great deal of interest in finding risk and preventative factors for age-related cognitive decline and AD. Of particular interest are those factors that are modifiable, because interventions that decrease the effect of a risk factor or facilitate a preventative factor could potentially delay the onset of the disease or prevent it altogether.
Dietary enrichment reduces cognitive dysfunction in dogs. Investigators recently studied the effects of an enriched diet on age-related cognitive decline in dogs, a model that mimics the behavioral and brain pathological declines of older humans more closely than rodent models. Young and old dogs were given a series of baseline cognitive tests. Half of each age group then remained on a standard dog chow diet, while the other half of each age group was placed on a diet enriched with antioxidants and mitochondrial co-factors, which improve nerve cell energy and efficiency and decrease production of molecules that contribute to oxidative damage in the brain. Animals remained on their respective diets for six months and then were assessed again for cognitive performance on a variety of tasks. When tested, old dogs on the control diet learned more slowly than the young dogs and made significantly more errors; however, when compared to the old animals on the control diet, old animals on the enriched diet showed significantly better learning, although not to the level of the younger animals. The enriched diet had minimal effect on the behavioral performance of young dogs. The success of this simple, cost-effective intervention has significant implications for dietary interventions that might lessen or even prevent some of the cognitive decline seen with age and with disease, and a pilot trial in humans is currently underway.
Animal Models of Neurodegenerative Disease
Animal models that mimic human disease are central to research for many reasons. Animals and humans share many genetic and physiologic features, so experimental results obtained in animals can frequently (although not always) be extrapolated to humans. It is much easier to create specific genetic mutations and observe their effects in animals than to search for them in humans, and because the lifespan of most animals is relatively short, it is easier to observe the effects of those mutations over several generations.
A new mouse model sheds light on AD pathology. Researchers recently developed the first mouse model that displays the primary neuropathological features of AD – the development of amyloid plaques and neurofibrillary tangles (NFTs) – as well as synaptic loss in the central nervous system, which in humans correlates best with the cognitive declines observed in AD. Of particular interest to scientists is the novel method – microinjection of mutant amyloid precursor protein (APP) and tau transgenes into single-cell mouse mutant embryos – used to develop this “triple transgenic” model. Studies in the new model suggest that synaptic dysfunction actually occurs before extracellular amyloid deposition and NFT development, and coincides with the accumulation of intraneuronal amyloid. This model may enable a more complete understanding of AD etiology, as well as improved evaluation of new diagnostic techniques and treatments.
Pre-clinical Research
There are currently no effective, generally useful treatments for AD – i.e., a treatment that works on large numbers of patients, that maintains its effectiveness for a long period, that works in both early and late stages of the disease, that improves functioning of patients in activities of daily living as well as performance on sensitive neuropsychological measurements, and that has no serious side effects. In addition, none of the treatments presently approved for AD alter the progressive underlying pathology of the disease. One way to treat the disease successfully may be to interfere with early pathological changes in the brain, including the development of amyloid deposits and the formation of neurofibrillary tangles. A number of promising approaches are currently being developed and tested in model systems; if these approaches prove safe and effective in animals, studies in humans could follow.
New gene therapy approach for treatment of neurodegenerative disorders. In two recent studies, investigators used genetically modified viruses to attack features of AD and amyotrophic lateral sclerosis (ALS), a disease in which motor neurons in the spinal cord degenerate, leading to muscle paralysis and death. In one study, a virus carrying the human neprilysin gene, which makes an enzyme that degrades beta amyloid, was injected into brain areas containing amyloid plaques in mice that were genetically modified to develop AD pathology. The production of neprilysin appeared to increase degradation or reduce the growth of existing plaques; in fact, the plaque load was reduced to less than half that found in untreated areas. In the second study, researchers modified a virus to produce one of two growth factors, insulin growth factor 1 (IGF-1) or glial cell line-derived neurotrophic factor (GDNF). These vectors were then injected into muscles of ALS transgenic mice either before or at the time of disease onset. The virus was taken up by nerves in the muscle and transported to the spinal cord, where the growth factor was produced. The researchers found that delivery of IGF-1 delayed the onset and rate of progression of the disease when given before onset of symptoms, and extended lifespan and delayed motor functional decline when given at disease onset. GDNF was less effective in these experiments. These two studies suggest that boosting normal protective processes in the nervous system might help prevent or treat degeneration associated with AD or ALS. Both studies offer new hope for the treatment of these disorders.
NSAIDS reduce amyloid ß-peptide formation in culture and in animal models. Evidence from epidemiological and clinical studies shows a correlation between a decreased risk of developing AD and long-term use of non-steroidal anti-inflammatory drugs (NSAIDs), suggesting that inflammatory processes may be involved in AD pathophysiology. Investigators recently studied the effects of twenty clinically used NSAIDs on Aß1-42, a particularly toxic form of amyloid, on cells in culture and in animal models of AD. Eight of the compounds significantly lowered Aß1-42 levels both in vitro and in the mice. These drugs worked through a mechanism independent of cyclooxygenase inhibition, the primary anti-inflammatory action of most NSAIDs; in fact, the researchers suggest that these eight NSAIDs might delay accumulation of toxic amyloid by inhibition of ?-secretase, a key enzyme in the development of Aß1-42. The researchers also found that newer specific cyclooxygenase-2 inhibitors, in wide use and with fewer side effects, do not lower Aß1-42 levels in vitro or in vivo. These studies identify a novel neuroprotective mechanism of some NSAIDs and suggest that it may be useful to screen new agents for their actions on a key biochemical process in AD neuropathology, although whether or not the amyloid-lowering drugs or others will be effective in clinical trials is yet to be determined.
Clinical Trials
Today, the few FDA-approved drug treatments for AD maintain cognitive function in AD patients in only a subset of patients and for only a limited time. However, an estimated 30 compounds are presently or will soon be tested in human AD clinical trials. These studies are sponsored by a number of sources, including the NIA, other NIH institutes, and the private sector, primarily pharmaceutical companies. Compounds now under scrutiny focus on three major areas of treatment: Short-term maintenance of cognitive function; slowing the progress of the disease, delaying AD’s onset, or preventing the disease altogether; and managing behavioral problems associated with AD.
Interest is currently focusing on compounds that directly target disease-related pathologies. An important research focus is in prevention trials, and a number are underway to test the effectiveness of therapies in people without symptoms or who have only slight memory problems. The first NIH AD prevention trial is currently under way at more than 70 sites across the U.S. This trial compares the effects of vitamin E and donepezil (brand name Aricept) in preventing AD in people diagnosed with mild cognitive impairment. Further examination of estrogen and studies of various classes of anti-inflammatory drugs and antioxidants are also ongoing, and as scientists test these currently available medications, the next generation of drugs is being developed, targeting specific abnormal cellular pathways, including plaque and tangle formation and death of brain cells. Prevention trials are among the most costly of research projects, but, if successful, the payoff in terms of reduced disease and disability will be significant.
Caregiving of AD Patients
Most of the approximately 4 million Americans with AD today are cared for outside the institutional setting by an adult child or in-law, a spouse, another relative, or a friend. Caregivers frequently experience significant emotional stress, physical strain, and financial burdens, yet they often do not receive adequate support. Several recent studies have explored the problems faced by caregivers of AD patients, as well as possible interventions to reduce their burdens.
End-of-life care stresses caregivers of dementia patients. Although family caregiving has been extensively studied, there has been less research on the impact of end-of-life care on caregivers who are family members of persons with dementia or to the caregivers' responses to the death of the patient. As part of the NIA’s Resources for Enhancing Alzheimer’s Caregiver Health (REACH) study, a multisite randomized clinical intervention of 1222 caregiver and recipient dyads, investigators assessed the type and intensity of care provided by 217 family caregivers to persons with dementia during the year before the patient's death, as well as the caregivers' responses to the death. Additionally, this group was compared to the 180 caregivers who institutionalized their family member. The researchers found that the in-home caregivers reported tremendous levels of stress in the year leading up to the care recipient’s death, and that levels of caregiver depression “spiked” immediately following the care recipient death. However, the caregivers in this study demonstrated tremendous resilience: Within fifteen weeks of the recipient’s death, depression returned to pre-death levels, and within one year, depression was significantly lower than pre-care recipient death levels. Importantly, caregiver depression for those placing their loved ones in an institution were slightly higher both pre- and post-death than for those caring for the patient at home.
Selected Future Research Directions in AD and the Neuroscience of Aging
Advances in neuroimaging have the potential to transform the way we predict, diagnose, monitor, and even treat mild cognitive impairment and AD. The NIA is currently developing an Alzheimer’s Disease Neuroimaging Initiative, a longitudinal, prospective, natural history study of normal aging, mild cognitive impairment, and early AD to evaluate neuroimaging techniques (e.g. MRI, PET) and other potential biomarkers of the disease. Biomarkers may decrease the time and cost of clinical trials, which increases the safety and efficiency of drug development. An important aspect of this initiative is that the clinical, imaging, and biological data and samples will be made available promptly to all qualified scientific investigators in academic as well as industrial research communities. The initiative is planned as a partnership among the NIA/NIH and several other private and government organizations.
The NIA is accelerating the pace of Alzheimer’s disease genetics research with its AD Genetics Initiative, a major new program to speed the process of creating a large repository of DNA and cell lines from families with multiple AD cases. The goal of this initiative is to develop the resources necessary for identifying the remaining late-onset AD (LOAD) risk factor genes, associated environmental factors, and the interactions of genes and the environment. The AD Genetics Initiative will intensify sample collection and encourage data sharing by providing access to the repository to qualified investigators.
Reducing Disease And Disability
Chronic disease and disability can compromise the quality of life for older people. Some 79 percent of people age 70 and older have at least one of seven potentially disabling chronic conditions (arthritis, hypertension, heart disease, diabetes, respiratory diseases, stroke, and cancer).6 The burden of such chronic conditions is felt not only by individuals but also by families, employers, and the health care system. Research to improve understanding of the risk and protective factors for chronic disease and disability can lead to the development of effective prevention strategies.
Treatment and Prevention of Disease
Treatment of any specific disease in older people can be complicated by the presence of other diseases and disorders and by the concomitant use of multiple medications to treat various conditions. Potential interactions of medications, including those of prescribed drugs with over-the-counter drugs and dietary supplements, represent additional concerns. Moreover, adherence to treatment regimens can be difficult, as older patients often must maintain a complex medication schedule. Research is ongoing to determine the best treatment approaches for older patients, particularly those with concurrent medical conditions, and to identify strategies for improving adherence and minimizing potentially adverse effects of medications.
Newly defined pathway for steroid action on cells provides the means to reverse osteoporosis in mice. It is estimated that over 10 million men and women currently have osteoporosis and an additional 18 million have low bone mass and are at risk.7 Treatment options for women have most often included hormone replacement therapy involving estrogen; however, recent clinical evidence has indicated that estrogen replacement therapy may have undesired side effects on other organs.
Investigators have outlined a previously unrecognized pathway for estrogen action through a receptor at the cell surface, with subsequent steps to influence a set of genes in the nucleus that had not been known to be involved with estrogen action. In addition, they found that this pathway of action is also used by androgen receptors and that a synthetic compound, 4-estren-3a, 17ß-diol, can activate both estrogen and androgen receptor pathways. Finally, studies in both male and female mice demonstrate that this mechanism of action by estren can be used not only to preserve bone in animals lacking estrogen, but also to increase bone mass without stimulating unwanted effects in reproductive organs. This work has already had far-reaching effects in bone and steroid biology and provides an entirely new pathway for future clinical investigation to not only stop progression of bone loss, but reverse bone loss without dangerous side effects.
Mimicking caloric restriction to increase longevity in animal models. Reducing calorie intake increases longevity and delays adverse age-related changes in a variety of organisms. However, the mechanism whereby caloric restriction works remains unknown, thus preventing rational development of biological interventions to produce the effect provided by actually restricting calories. Several recently discovered genetic and pharmacological interventions are providing clues about ways to mimic caloric restriction, and are indirectly leading to a better understanding of the mechanism of caloric restriction. For example:
- Researchers have identified a fruit fly gene called Indy (“I’m not dead yet”), which, when mutated, doubles the life span of the fly without apparent ill effects. The normal Indy gene is involved in the transport of certain nutrients between cells; when mutated, this transport is dramatically slowed, delivery of the nutrients to the cells is restricted, and overall energy metabolism is reduced.
- Studies in yeast provide another perspective on the mechanism of caloric restriction. Investigators have found that several compounds that increase activity of the enzyme sir2 in yeast also increase their mean longevity. The most potent activator of sir2 identified so far is resveratrol, a compound found in many foods, especially red wine. The investigators postulate that sir2 expression and activity mimic the mild stress induced in yeast by caloric restriction. The possible benefits of resveratrol in the human diet have been noted previously, but the mechanism of its beneficial actions was not known.
Intensity of warfarin therapy affects stroke severity and mortality in older patients with atrial fibrillation. Atrial fibrillation (AF) is a disorder of heart rhythm whose frequency increases greatly with advancing age. Research has shown that the incidence of ischemic stroke (stroke caused by blood clots) among older patients with AF is greatly reduced by treatment with warfarin, a drug used to control and prevent blood clotting disorders. This therapy helps maintain an International Normalized Ratio (INR) (an index of anticoagulation intensity) value of 2.0 or greater. However, warfarin’s effect on the severity of stroke and stroke-related mortality is less well known. Researchers examined ischemic stroke outcomes in a large group of older patients with AF. They found that patients who received anticoagulation therapy resulting in an INR value of 2.0 or greater at the time of stroke were likely to have less severe neurologic damage than patients who did not receive anticoagulation therapy, or who received therapy that resulted in an INR value of less than 2.0. Further, the 30-day mortality rate was significantly lower in patients who received warfarin resulting in an INR value of 2.0 or greater, compared with the mortality rate in patients who received warfarin resulting in an INR value of less than 2.0. Older individuals with AF are often treated less aggressively due to concern over possible adverse effects, but these findings emphasize the importance of maintaining adequate anticoagulation in this population.
Advances in the diagnosis and treatment of prion diseases. Prions are infectious proteins that transform a normal cellular protein (PrPC) into an abnormal virulent form (PrPSc) that accumulates in the central nervous system, producing fatal neurological disease characterized by sponge-like holes in the brain that result in progressive disturbances in movement, emotion, sleep, and cognition. Recent research has targeted the diagnosis and treatment of prion diseases. Commercially viable and automated diagnostic tests for bovine spongiform encephalopathy (BSE, or “mad cow disease”) and chronic wasting disease (CWD), a wildlife disease in North America, have recently been developed; the sensitivity of these new tests is at least 10 times greater than conventional bioassay methods for BSE and CWD. Investigators have also developed a series of derivatives of the anti-malarial drug quinacrine, which is currently under clinical evaluation for treatment of prion diseases. These compounds, which were engineered for increased potency, were both empirically tested and computer modeled to explore the maximal clearance of PrPSc from cells in tissue culture while minimizing cellular toxicity. Several compounds that were at least 10 times more effective than quinacrine were identified; these compounds may prove potent alternatives to quinacrine treatment.
Exendin-4 as a treatment for type 2 diabetes. NIH investigators searching for potential treatments for type 2 diabetes conducted a study of the compound exendin-4. This compound is an analog of the gut hormone GLP-1, which is naturally released after eating and which can lower blood sugar in people with diabetes when given in sufficient doses. Study participants received injections of the drug twice daily for a month. The investigators found that: 1) exendin-4 is well tolerated, 2) it retains efficacy for at least one month, 3) there were no unexpected side effects, and 4) it is at least as effective in lowering blood glucose as current treatments for type 2 diabetes. These results indicate that exendin 4-is a viable potential candidate agent for treating type 2 diabetes, and that phase 3 studies of the drug are appropriate.Story of Discovery: Multipotent Stem Cells and the Diseases of Aging diabetes, and that phase 3 studies of the drug are appropriate.
|
Story of Discovery: Multipotent Stem Cells and the Diseases of Aging
As we grow older, we become increasingly vulnerable to the development of many age-related chronic conditions, including Alzheimer’s disease, Parkinson’s disease, heart disease, and diabetes. These conditions, which are characterized by damage to and death of cells in various organs and systems, impair quality of life and are frequently fatal. However, we carry within ourselves the seeds of our own cellular renewal in the form of primitive cells, called stem cells, that have the potential to repair damaged tissue. Although stem cells appear to be present in numbers or in functional capacity that is insufficient to effectively combat some of the diseases of aging unaided, new research findings are suggesting ways to harness the power of these remarkable cells to treat a number of diseases and conditions.
A particularly exciting area of stem cell research is in “adult” stem cells. Adult stem cells have not yet differentiated into cells of a particular type (e.g., neural cells, blood cells, etc.), but they may be committed to doing so. For example, in the bone marrow, some stem cells give rise to red blood cells, white blood cells, and platelets, while others make bone cells and may be able to make additional cell types under appropriate conditions.
Researchers have known about stem cells since around the turn of the twentieth century, when European scientists made the momentous discovery that all blood cells derive from a single type of primitive cell. But it wasn’t until 1961 that researchers identified the properties of the hematopoietic stem cell B a groundbreaking finding that led to the development and refinement of the bone marrow transplant, which is today a widely-adopted treatment for certain types of leukemia and other blood diseases.
Today, stem cells have been identified in tissues throughout the body, including the blood, the brain, the liver, the skin, and even the teeth, and new information is rapidly emerging about the properties of stem cells and their potential uses. For example, NIH-supported researchers recently identified, for the first time, a population of stem cells in the heart, and this finding provided important information about the underlying pathology of the diseased heart. Examining heart muscle tissue from older individuals with and without heart disease, they found that, in the normal heart, myocytes (the cells of the heart muscle) may lose their ability to replicate, but are replaced by cardiac stem cells. In the diseased heart, the stem cells do not adequately replace the lost myocytes, leading to an imbalance between cell death and regeneration that is associated with the development of pathology. The identification of cardiac stem cells suggests new avenues for myocardial repair, and provides important information about the pathogenesis of heart disease and failure.
Neural stem cells are of particular interest to the study of Alzheimer’s disease and other neurodegenerative diseases of aging. Through several recent studies, NIH-supported researchers have found that environmental cues, which vary among brain subregions, may determine the fate of a stem cell, that neurogenesis (or the formation of new neurons) may require the cooperation of multiple protein factors, and that neural stem-like cells from human brain tissue can form neurons.
In fact, several recent studies demonstrate that neural stem cells in the adult hippocampus, a brain area important for learning and memory, develop essential properties of functional neurons. The new neurons have properties similar to their mature neighbors; they receive input from other cells; they make functional connections, called synapses, with normal hippocampal neurons; and they release neurotransmitters, the chemical mediators of neuronal communication. Moreover, new neurons are made in the aged brain, and a recent study showed that once new neurons find a home in the hippocampus, they remain stable in numbers and location over time.
Whether adult stem cells from different sources can form several cell types, or can best replace cells of a particular type, they have enormous potential for cell-based therapies in disease. Clinical and preclinical research in this area is ongoing. In one recent, highly provocative study, mice in which heart damage had been induced were injected with cytokines (proteins) called stem cell factor (SCF) and granulocyte-colony-stimulating factor (G-CSF). Stimulated by the cytokines, primitive bone marrow cells swarmed to the hearts, converted to several different types of cardiac cells, and contributed to repair of the damaged tissue, improving both the heart function and the survival of the treated mice. This finding, while preliminary, suggests that it may be possible to mobilize the body’s own naturally-occurring stem cells to repair tissue damage and fight disease. Other potential applications include replacing dopamine-producing cells in the brains of Parkinson’s disease patients or developing insulin-producing cells for type I diabetes. Although there is currently no widespread clinical application of such treatments, the use of stem cells to combat the diseases of aging may be just around the corner. |
Molecular Understanding of Disease Processes
Gene expression profiling in Werner’s Syndrome (WS) closely resembles that of normal aging. WS is a condition in which younger patients display a number of the clinical signs and symptoms usually associated with normal aging. To assess whether WS features are related to the same genetic influences as normal aging, investigators characterized the expression of 6,912 genes derived from young donors, old donors, and WS patients. Of the analyzed genes, 6.3 percent displayed significant differences in expression when either WS or old donor cells were compared to young donor cells. Expression alterations in WS were also strikingly similar to those in normal aging. These results suggest that a difference in the expression of certain genes could produce many of the complex clinical features of WS. The remarkable similarity between WS and normal aging suggests that WS is associated with the acceleration of a normal aging mechanism. This supports the use of WS as an aging model, and identifies genes that may be important in aging.
“Fat Genes” in the worm may shed light on human obesity. Data from the Centers for Disease Control indicate that among U.S. adults ages 20-74, 35 percent are overweight (defined as having a body mass index, or BMI, of 25.0-29.9), and some 27 percent are clinically obese (BMI is 30.0 or above).8 Overweight and obesity are associated with an array of health problems, including heart disease, stroke, osteoarthritis, adult-onset diabetes, and certain types of cancer. Although behavioral and environmental factors are the primary contributors to overweight and obesity, heredity plays a significant role in determining individual susceptibility to these conditions, and genes influence how the body burns calories for energy and stores fat. NIH-supported researchers recently searched for genes necessary for fat storage in C. elegans, a tiny worm that is frequently used in genetic studies. Using RNA interference (RNAi), a technique in which genes are inactivated one at a time to determine their function, the investigators screened the 16,757 genes in the C. elegans genome and found 417 genes involved in fat storage. Inactivation of 305 genes caused reduced body fat, and inactivation of 112 genes increased fat storage. Many of these C. elegans fat regulatory genes have human counterparts, a number of which have not been previously implicated in the regulation of fat storage. The fat regulation genes identified in C. elegans may suggest new targets for treating human obesity and its associated diseases.
Age-associated alternations in mitochondrial function are implicated in insulin resistance in older persons. Insulin resistance is a metabolic disorder that can occur with advancing age and is thought to precede the development of type II diabetes in older adults. The underlying disease process of type II diabetes among older persons is not yet known. However, researchers hypothesize that age-related increases in the fat content of muscle may play an important role in the development of insulin resistance, and that age-related changes in the function of mitochondria (the cells’ “energy centers”) may be responsible for this accumulation. In a recent study, investigators used nuclear magnetic resonance (NMR) spectroscopy, a technology that allows non-invasive quantification of fat content in tissues and can also measure muscle metabolism, to determine whether insulin resistance in older persons is associated with increased fat content within muscle. The participants consisted of healthy older and young individuals who were matched by their muscle mass and body fat content. The older study participants were significantly insulin-resistant compared to the young, mainly due to increased muscle insulin resistance. NMR spectroscopy data revealed increased fat content and reduced mitochondrial activity in muscle. These results indicate an association between altered mitochondrial function in humans and an accumulation of fat within muscle that in turn leads to insulin resistance.
Biology Of Aging
Aging is accompanied by gradual changes in most body systems. Research on the biology of aging focuses on understanding the cellular and molecular processes underlying these changes as well as those accompanying the onset of age-related diseases. As scientists learn more about these processes, experiments can be designed to understand when and how pathological changes begin, providing important clues toward developing interventions to prevent or treat disease. A great deal has been learned about structural and functional changes that occur in different body systems. Research has expanded our knowledge, too, of the biologic factors associated with extended longevity in humans and animal models.
Extending the Lifespan
Identification of the factors that affect the overall lifespan of an organism will help us better understand the aging process, and will also help us develop interventions to keep older people healthy and free of disease and/or disability as long as possible. Over the last ten years, the NIA Longevity Assurance Gene (LAG) Initiative has been pivotal in the identification of multiple genes, pathways, and biological processes involved in the regulation of longevity and aging in multiple organisms (yeast, nematode, fruit fly, mouse, human). Through the use of both invertebrate and mammalian models, the LAG Initiative has identified common factors and mechanisms that mediate longevity and extend health span.
Scientific advances in model systems provide the critical scientific foundation to extend NIA-supported studies to humans. In addition, they provide the knowledge base necessary to guide the rational development and testing of intervention strategies to delay aging, promote longevity, and extend human health span in the near future. For example, researchers recently used RNA interference (RNAi), a technique to inactivate individual genes one at a time, to identify genes involved in longevity regulation in the worm C. elegans. They found that inactivation of many genes involved in mitochondrial function extended longevity; in fact, 15 per cent of the genes influencing longevity were specific for mitochondrial function. These results reinforce the idea that energy metabolism is important in determining animal longevity.
Increased evidence of familial and genetic factors in exceptionally long and healthy life. Three recent studies of exceptionally long-lived individuals and their children suggest that a tendency for exceptionally long and healthy life to run in families may be related to exceptionally favorable risk factor profiles for cardiovascular and other diseases over the life span. In the first study, middle-aged sons of long-lived parents had lower systolic pressures, better cholesterol levels, and decreased frequencies of the APOE-e4 allele (a gene variant commonly associated with cardiovascular disease and Alzheimer’s disease) compared to middle-aged sons of shorter-lived parents. The second group found that compared to controls, children of centenarians had markedly reduced prevalence of some age-related diseases, including heart disease, hypertension, and diabetes. In the third study, researchers found that Ashkenazi Jewish centenarians and their offspring were more likely than a control group to have a variant form of a gene for a cholesterol regulating protein. This form of the gene is associated with larger-than-average cholesterol-carrying particles in the blood, and with higher levels of HDL (“good”) cholesterol, both of which were found in the centenarians’ offspring. These findings suggest that larger lipoprotein particle sizes may be one of the familial factors that promote long and healthy survival.
Together these findings add to growing evidence that the good health profiles that occur in centenarians and their children differ markedly from age-matched counterparts in the general population, and that there are familial and possibly genetic components that most likely influence protective factors against age-related disease and promote exceptionally healthy human survival. Identification of these factors earlier in life could lead to new interventions to prevent age-related diseases and disabilities, and extend healthy lifespan.
Selected Future Research Directions in the Biology of Aging
The identification of “longevity genes” is complex and necessarily interdisciplinary, involving ongoing interactions between basic and epidemiologic researchers to accelerate discovery of and confirm translational findings. To facilitate identification and understanding of longevity genes, the NIA has formed a Longevity Consortium, a self-sufficient system for rapid generation, review, and funding of new projects. Components of the Consortium include:
- Multiple basic laboratories addressing relevant disciplines including cell and molecular biology, physiology, and biochemistry
- A collaborative group of major epidemiologic studies with data on multiple outcomes in established study populations
- Diverse populations and large sample sizes to allow analyses of subgroups and covariates
- Registry and/or database capacity to allow rapid identification of possible cases and controls, and genotype and phenotype information
- Genotyping, genomics, computational, and cell line repository facilities to allow standardization and economies of scale
- System for rapid information exchange among basic and epidemiologic researchers to convey new findings and conduct follow-up studies
Members of the Consortium include epidemiologists, geneticists, population biologists, statisticians, and others with an interest in the genetic and molecular basis for longevity, and the Consortium draws on the study populations of 15 of the largest human aging studies, including the Cardiovascular Health Study, the Women’s Health Initiative, Health ABC, the Study of Osteoporotic Fractures, the Rotterdam Study, the Honolulu Heart Study, and the New England Centenarian Study. Altogether, Consortium researchers will have access to data on some 200,000 study subjects.
Behavioral And Social Research
Behavioral and lifestyle factors have a profound impact on health throughout the life span. For example, older adults can help to prevent disease and disability and improve their quality of life through healthy behaviors such as ensuring proper nutrition, exercise, use of preventive health care, and avoiding smoking and alcohol abuse. NIA research on behavioral and social factors in aging encompasses a number of areas, including the effects of behavior and attitude on health, economic implications of aging at both the personal and societal levels, and the demographics of aging.
Does more spending buy better care? Dramatic differences in per-capita Medicare spending exist across U.S. regions. For example, in 1996, average per-capita Medicare spending in Miami was over two and a half times the average in Minneapolis. These regional differences in spending are due neither to differences in the prices of medical services nor to levels of illness or socioeconomic status; rather, they are largely due to the overall quantity or intensity of medical services in high-cost regions. Remarkably little is known, however, about whether the higher spending results in better medical care or better health outcomes.
In a recent study, researchers used a variety of databases and drew on recent advances in research methods to examine the relationship between increased Medicare spending and the content, quality, and outcomes of care. They assigned each of 306 U.S. regions to one of five different quintiles of spending using a measure of overall practice intensity. They then studied nearly one million Medicare enrollees who had been hospitalized in the mid 1990s for a heart attack, hip fracture, or new diagnosis of colorectal cancer, as well as a representative sample of the elderly, and found:
- Patients in the highest spending regions received about 60 percent more medical care than similar patients in the conservative regions, and the additional services (and thus higher spending) were largely due to a more inpatient-based and specialist-oriented pattern of practice.
- Residents of higher spending regions did not receive more major surgery.
- Access to basic health care services was slightly worse in high-spending regions.
- Quality of care was no better in the higher spending regions; for example, although mammography was performed equally across regions of different spending levels, other preventive services such as flu and pneumococcal immunizations and pap smears were performed less frequently in the highest spending regions.
- Finally, health outcomes were no better in higher spending regions: For example, during five years of follow-up mortality rates after adjustment for health status were two to five percent higher in the regions that provided more care.
These findings suggest that the quality and outcomes of care for Medicare enrollees are at least as good, and perhaps better, in lower-spending regions of the United States.
Health, life expectancy, and health care spending among the elderly. Life expectancy among the elderly has been improving for decades, and some economists have speculated that increasing longevity could be associated with higher healthcare costs. To determine whether this is true, investigators developed a model incorporating life expectancy, health status, and annual healthcare expenditures and found that a person with no functional limitations at age 70 could expect to live an additional 14.3 years and accumulate health expenditures of $136,000 (measured in 1998 dollars). Average expenditures per year increased with worsening health status, from about $4,600 per year for persons reporting no limitations to about $45,400 for institutionalized persons. These findings suggest that increased longevity is not in and of itself associated with higher healthcare costs, and that health promotion efforts, such as those encouraging smoking cessation and exercise, will not only result in better health and longer life for the elderly, but may also result in decreased costs for the healthcare system.
Does assistive technology substitute for personal assistance among the disabled elderly? Total expenditures in the United States for home care services amounted to more than $32 billion in 1999 and are expected to triple by 2010. However, researchers recently found that individuals who used assistive devices (such as canes, walkers, or wheelchairs), although more disabled, also received fewer hours of personal help per week than people who did not use such devices, indicating that assistive technologies can substitute to some degree for personal assistance. This suggests that compared to the financial costs of employing a paid personal assistant, use of assistive technology could amount to cost savings to both individuals and insurers.
Use of internet for healthcare information. Use of the Internet and e-mail for healthcare information has attracted considerable attention as a means to improve healthcare delivery. In fact, results from a recent national Internet-based survey of users ages 21 and older indicate that although Internet use is prevalent, it has not replaced traditional face-to-face contact between patient and provider. Specifically:
- Approximately 40 percent of respondents reported using the Internet to look for advice or information about health or healthcare.
- Six percent of respondents reported using email to contact their healthcare provider.
- One-third of respondents reported that the use of the Internet affected their healthcare decisions; however, despite these reports, the authors found few impacts of Internet usage on healthcare utilization or practices.
- Few individuals used the Internet to obtain prescription drugs.
- Individuals in worse health and with less education were more likely to use the Internet to contact others with similar health problems.
- Among individuals with more chronic healthcare conditions, Internet users gained a greater understanding of their condition from on-line health information.
- Few respondents changed their healthcare regime or made substantive decisions based on Internet information.
To respond to the unique needs of Internet users over 60, the NIH launched NIHSeniorHealth.gov on October 23, 2003. Developed by the NIA and the National Library of Medicine, this web site is easy for older adults to read, understand, remember, and navigate. For example, the site features large print and short, easy-to-read segments of information repeated in a variety of formats – such as open-captioned videos and short quizzes – to increase the likelihood it will be remembered. Consistent page layout and prompts help users move from one place to another on the site without feeling lost or overwhelmed. The site will also have a function that will allow users the option of reading the text or listening to it as it is read to them.
The risk of many diseases increases with age, so the site sponsors are focusing on health topics or specific diseases that are of particular interest to older people, including Alzheimer’s disease, Alzheimer’s disease caregiving, arthritis, balance problems, breast cancer, colorectal cancer, exercise for older adults, hearing loss, lung cancer, and prostate cancer. In coming months, topics will include complementary and alternative medicine, diabetes, falls, shingles, vision changes, and others. Each topic provides general background information, quizzes, frequently asked questions (FAQs), open-captioned video clips, transcripts for the videos, and photos and illustrations with captions. Since its launch, the site has averaged over 17,000 page requests per day.
NIHSeniorHealth.gov is expected to serve as a model for web designers seeking to make sites accessible for older adults. The NIA and NLM have also developed a booklet, Making Your Web Site Senior Friendly: A Checklist, which gives guidelines that can be used to update any web site with cognitive aspects of aging in mind.
Health Disparities
The health status of racial and ethnic minority groups in the U.S. has improved steadily over the last century. Despite such progress, disturbing disparities in health persist between majority and minority populations. Demographic projections predict a substantial change in the racial and ethnic makeup of the older population, heightening the need to examine and reduce differences in health and life expectancy. Research to date has shown that health disparities are associated with a broad, complex, and interrelated array of factors: Disease risk, diagnosis, progression, response to treatment, caregiving, access to care, and overall quality of life each may be affected by variables such as race, ethnicity, gender, socioeconomic status, age, education, occupation, country of origin, and possibly other lifetime and lifestyle differences. In one recent study, investigators found that adjusting for reading recognition scores on an achievement test known as the WRAT-3 attenuated racial group differences on most cognitive tests between older white and African Americans matched on years of education. The WRAT-3 scores served as an estimate of quality of education, and this finding suggests that it is quality and not necessarily years of education that may affect cognition in later years.
The NIA is committed to addressing health disparities through its research programs. For example, Satellite Diagnostic and Treatment Centers, part of the national Alzheimer’s Disease Centers (ADC) Program, have successfully recruited African Americans, Hispanics, Native Americans, and American Indian/Alaska Natives to AD prevention and treatment studies. Researchers on the NIA’s Religious Orders Study have made a major effort to enroll African American members of the Catholic clergy; the nature of the study population enables the etiology and pathology of AD to be established among individuals with similar educations, occupations, socioeconomic status, and lifestyles.
The NIA is also participating with the National Institute of Environmental Health Sciences, the National Cancer Institute, and the NIH Office of Behavioral and Social Sciences Research on new Centers for Population Health and Health Disparities, which are designed to support interdisciplinary research to examine how the social and physical environment, behavioral factors, and biologic pathways interact to determine health and disease in populations. To date, NIH has awarded eight grants in total from this initiative. NIA is supporting two: A series of inter-related studies involving a cohort of older adults of Puerto Rican origin in the Boston area, with particular attention to specific stressors affecting that community, determining the effect of these stressors on allostatic load (“wear and tear” on body systems resulting from stress) and, in turn, on disease-specific outcomes, and an analysis of data from the Health and Retirement Study and the Panel Study of Income Dynamics to determine neighborhood factors that impact the functional and cognitive aspects of the disabling process in the elderly. The latter project is coordinated with an NIEHS-awarded Center grant.
Where a patient lives determines likelihood of knee surgery. Researchers used Medicare fee-for-service claims data for 1998-2000 to determine the incidence of knee arthroplasty between Hospital Referral Regions by sex and race or ethnic group. They found that in some regions, the rate of knee arthroplasty for black women was significantly lower than that for white women (e.g., Washington DC; Atlanta; Chicago), whereas in other regions (e.g., Los Angeles; Manhattan; Birmingham), the rates were roughly equal. Around 35 percent of the national differences in arthroplasty rates for black women and 95 percent of the national differences for Hispanic women are explained by the fact that black and Hispanic women are more likely to live in regions with lower rates for all races and ethnic groups. In the study, residential segregation by race (among black women) and low income (among Hispanic women and black men) were associated with larger differences in arthroplasty rates. Furthermore, arthroplasty rates were consistently lower among black men than among white men in nearly every region, and in some areas the rates for black men were less than one third those for white men. These persistent differences cannot be explained on the basis of financial or geographic barriers alone, since the pattern was not apparent for black women living in the same neighborhoods. These variations underscore the importance of examining geography and sex in determining racial or ethnic barriers to health care.
Contributions of major diseases to disparities in mortality. Researchers in a recent study found that although many conditions contribute to socioeconomic and racial disparities in potential life-years lost, a few conditions account for most of these disparities – smoking-related diseases in the case of mortality among persons with fewer years of education, and hypertension, HIV, diabetes mellitus, and trauma in the case of mortality among black persons. These findings have important implications for targeting efforts to reduce existing disparities in mortality rates.
Selected Future Research Directions in Health Disparities
The NIA has implemented Healthy Aging in Neighborhoods of Diversity Across the Lifespan (HANDLS), a community-based study of health disparities among different racial, ethnic, and socioeconomic groups in Baltimore. The purpose of HANDLS is to disentangle the effects of race and SES on risk factors for morbidity and mortality, incidence and progression of pre-clinical disease, development and persistence of health disparities, and longitudinal health status and health risks. Unique to the HANDLS study is the use of two fully-equipped mobile research laboratories (MRVs) that enable investigators to collect data directly in the neighborhoods under study, establishing links with the community and increasing both the interest of potential participants and the retention rate. The pilot phase of the study was completed in December 2001, and the full-scope study is now being planned for implementation in 2004-2005.
Conclusion
As our population rapidly grows older, it is ever more urgent that we find effective ways to address the often devastating diseases and conditions associated with advanced age. Since the NIA’s founding in 1974, groundwork has been laid for today’s important advances in understanding basic aging, preventing disease and disability, including AD, and defining special social and behavioral issues for older individuals, their families and caregivers, and clinicians. The latest studies provide additional basic understandings as well as improved interventions to treat and even prevent some of the more devastating and disabling aspects of aging. With such research continued and intensified, we can move forward in meeting the promise of a healthy old age by improving the health and well being of older people in America.
Budget Policy
The Fiscal Year 2005 budget request for the NIA is $1,055,666,000, an increase of $31,068,000 and 3 percent over the FY 2004 Final Conference Level. Also included in the FY 2005 request, is NIA’s support for the trans-NIH Roadmap initiatives, estimated at 0.63% of the FY 2005 budget request. This Roadmap funding is distributed through the mechanisms of support, consistent with the anticipated funding for the Roadmap initiatives. A full description of this trans-NIH program may be found in the NIH Overview.
A five year history of FTEs and Funding Levels for NIA are shown in the graphs below. Note that the Fiscal Year 2001 FTE figure is not comparable to the figures in the succeeding years due to NIH’s consolidation of its Human Resources function in FY 2003.
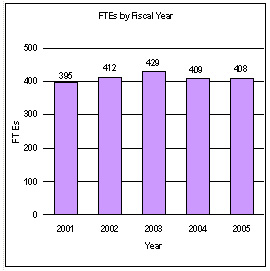
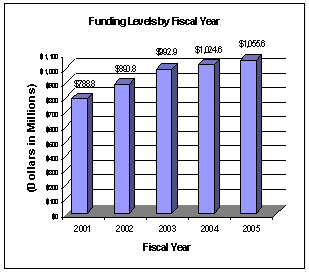
NIH’s highest priority is the funding of medical research through research project grants (RPGs). Support for RPGs allows NIH to sustain the scientific momentum of investigator-initiated research while providing new research opportunities. The FY 2005 NIH request provides for an aggregate 1.3 percent increase in average cost for Research Project Grants, consistent with the Gross Domestic Product deflator. The NIA is providing an average cost increase of 1.9 percent for direct recurring costs in noncompeting continuation awards. Competing RPGs are based on an average cost increase of 1 percent.
Advancement in medical research is dependent on maintaining the supply of new investigators with new ideas. In the Fiscal Year 2005 request, NIA will support 584 pre- and postdoctoral trainees in full-time training positions. Stipend levels for pre-doctoral and post-doctoral recipients supported through the Ruth L. Kirschstein National Research Service Awards will remain at FY 2004 levels.
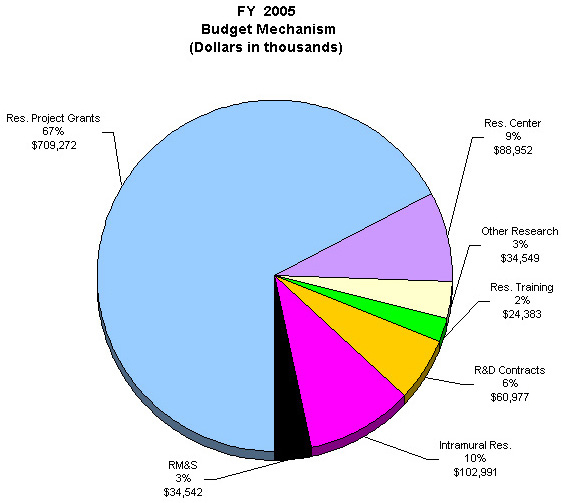
The Fiscal Year 2005 request includes funding for 67 research centers, 231 other research grants, including 204 clinical career awards, and 112 R&D contracts. Intramural Research and Research Management and Support receive increases to support increased pay and estimated inflationary increases in FY 2005.
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2000: Key Indicators of Well-Being. 2000.
- See “Handout on Health: Osteoarthritis,” National Institute of Arthritis and Musculoskeletal and Skin Diseases, July 2002.
- See America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. National Osteoporosis Foundation, February 2002.
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, and Evans DA: Alzheimer disease in the US population: prevalence estimates using the 2000 Census. Arch Neurol 60: 1119-22, 2003.
- Ibid.
- National Center for Health Statistics. Health, United States, 1999 With Health and Aging Chartbook. Figure 11, pg. 41. Hyattsville, MD: 1999.
- “Osteoporosis: Progress and Promise.” National Institute of Arthritis and Musculoskeletal and Skin Diseases, 2000.
- “Defining Overweight and Obesity.” National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/nccdphp/dnpa/obesity/defining.htm.
