Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended. Reauthorizing legislation will be submitted.
Budget Authority
|
FY 1999
Actual |
FY 2000
Estimate |
FY 2001
Estimate |
Increase or
Decrease |
|
FTE |
Amount |
FTE |
Amount |
FTE |
Amount |
FTE |
Amount |
|
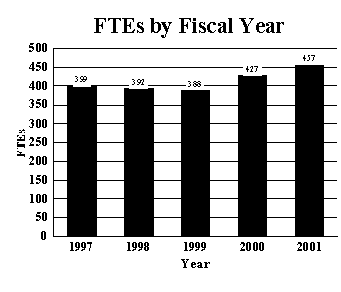
388 |
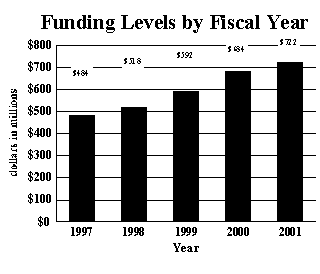
$597,652,000 |
427 |
$683,718,000 |
457 |
$721,651,000 |
30 |
+$37,933,000 |
This document provides justification for the FY 2001 non-AIDS activities of the National Institute on Aging (NIA). Justification of NIH-wide FY 2001 AIDS activities can be found in the NIH section entitled "Office of AIDS Research (OAR)."
Since 1974, the National Institute on Aging (NIA) has led a national scientific effort to understand the nature of aging and to extend the healthy, active years of life for all Americans. This enterprise has rapidly expanded knowledge about the biological, behavioral, and social changes that occur with advancing age and has disproved stereotypes of inevitable decline as people grow older. Recent findings have revealed dramatic and unexpected reductions in rates of disability among older persons compared to projected levels. Researchers have also generated effective strategies that can maintain or even enhance older people’s physical and mental abilities. It is clear that much old age disability can be prevented. These advances have contributed to progress in public health, health care, and disease prevention, and are beneficial to people of all ages.
Since the beginning of the 20th century, life expectancy at birth in the U.S. has increased from less than 50 years to more than 76 years(1). The challenge for the 21st century will be to make these added years as healthy and productive as possible and to maintain the current trend of decline in disability. There is no time to lose in discovering how to age well. Only 12 years from now, 75 million babyboomers, people born between 1946 and 1964, will begin to turn 65. By the middle of the next century, the number of Americans over the age of 65 will double, and the number of Americans over age 85 will increase fivefold or more, placing a significantly greater number of people at risk for disease and disability(2). It is urgent to develop more effective treatments for age-related diseases, including Alzheimer’s disease (AD), heart and vascular diseases, and cancer, and to prevent or delay the onset of disease and disability among older persons. Evidence shows that health research is making progress toward these goals.
During 1999, the NIA launched a nationwide Alzheimer’s disease (AD) prevention study, the Memory Impairment Study, that targets individuals with mild cognitive impairment. People with this newly recognized condition have a memory deficit beyond that expected for their age and education yet do not have other clinical signs of dementia. They also have a higher-than-normal chance of developing AD. The Memory Impairment Study will examine whether early
treatment of these relatively healthy and well-functioning people either with vitamin E (alpha-tocopherol) or donapezil (Aricept) can prevent further cognitive decline, including development of AD.
As the NIA celebrates its 25th anniversary, NIA staff have been working with scientific organizations, public and private groups, and the National Advisory Council on Aging to develop a five-year strategic plan for aging research that will help communicate the Institute’s future research activities. This collaboration is proving helpful both in creating new objectives and in forging partnerships to implement them. The plan is expected to be presented early in 2000.
The following narrative includes stories of discovery on Alzheimer’s disease and on the benefits of exercise. Selected research advances are discussed under each of four main topic headings: Alzheimer’s Disease and the Neuroscience of Aging, Reducing Chronic Disease and Disability, Biology of Aging, and Behavioral and Social Research. The final section describes future research directions for selected topics in aging research.
Alzheimer's Disease and the Neuroscience of Aging
Alzheimer’s disease, the most common cause of dementia, is the result of abnormal changes in the brain that lead to a precipitous decline in intellectual abilities and changes in behavior and personality. As the primary Federal agency responsible for research on Alzheimer’s disease, the NIA leads national efforts to gain greater understanding of the biological mechanisms underlying Alzheimer’s disease and to develop preventive measures and treatments based on research findings. Tragically, as many as four million Americans now suffer from Alzheimer’s disease (3), and the predicted explosive growth in the number of people living to 85 years and older, persons most at risk for dementia, lends an urgency to this research. Although the early signs of Alzheimer’s disease involve mild forgetfulness, the progressive dementia ultimately leaves patients incapable of caring for themselves. Behavior changes may cause patients to become agitated, sometimes to the point of causing harm to themselves or others. Alzheimer’s disease devastates its victims and profoundly affects the millions of family members and other loved ones who provide most of the care for people with this disease. Alzheimer’s disease also necessitates formal services at substantial cost to individuals and public programs, estimated at greater than $100 billion per year(4). While much remains to be done, research progress has been accelerating rapidly, bringing the field to the threshold of prevention trials.
In 1906, Dr. Alois Alzheimer described a patient, Auguste D., who experienced a four-year progressive decline into dementia and then died at age 55. Alzheimer’s postmortem study of Auguste D.’s brain revealed two striking pathological findings—neuritic plaques and neurofibrillary tangles. Decades later, these lesions, recognized as hallmarks of Alzheimer’s disease (AD), are the focus of a vigorous research effort to understand the underlying causes of dementia in late life and to develop compounds that will prevent the disease or block its progress.
For years, evidence has burgeoned about the protein fragment beta-amyloid in AD pathology. Beta-amyloid is the primary component of neuritic plaques, along with inflammatory cells and other insoluble filamentous proteins that can contribute to neuronal damage. An early and consistent feature in the AD brain, beta-amyloid surrounds brain cells (neurons) in regions of the brain involved in memory and cognition. Beta-amyloid peptide is derived from a much larger protein called the amyloid precursor protein (APP). The discovery in 1990 of a genetic mutation on chromosome 21 that ties APP to AD was the first real indication of a link between beta-amyloid production and the pathology of the disease. This mutation is associated with a rare form of early-onset, familial AD. Two other genes that cause early-onset AD were identified in 1995, presenilin-1 on chromosome 14 and presenilin-2 on chromosome 1. The proteins produced by the presenilin genes are tied to beta-amyloid by the discovery that the AD-linked mutations in these genes are associated with increased production of beta amyloid. A defect in any one of these three genes can cause AD, accounting for approximately 50% of the inherited early-onset cases, or as few as 10% of persons with AD.
For most individuals, however, susceptibility to AD is more complex, and its genetic component probably involves more than one gene. The only accepted risk factor for the common, late-onset form of AD is ApoE4, a variant of the ApoE gene on chromosome 19. ApoE4 accounts for approximately 50% of the genetic effect in the development of late-onset AD. Recent findings suggest that ApoE is critical for promoting beta-amyloid deposition in neuritic plaques. This and other evidence help define beta-amyloid as a prime target for intervention in the cascade of events that initiate neuronal degeneration.
An important recent advance in AD research was the generation of transgenic mice expressing the mutant forms of human beta-amyloid associated with early onset AD. These mice develop amyloid plaques with similarities to those observed in AD patients, providing for the first time a candidate mouse model of this disease. This year, scientists at the Elan Corporation immunized young beta-amyloid transgenic mice with a synthetic form of beta-amyloid found in plaques and succeeded in preventing almost entirely the deposition of beta-amyloid in mouse brains and reducing other features of disease compared with controls. Older transgenic mice vaccinated at 11 months also had a considerable reduction in amyloid deposition at 15 and 18 months when compared with controls. Although the relevance of this model to human disease remains uncertain, these results raise the possibility of immunization as a treatment or perhaps a prophylactic measure against AD.
The role of neurofibrillary tangles, the second characteristic lesion of AD, has also been the subject of recent research advances. Found in the same areas of diseased brains as plaques, but inside neurons, tangles are the wreckage of the cell’s internal structural support and nutrient transportation system. In healthy cells, microtubules are formed like train tracks—long, parallel rails stabilized by "railroad ties" consisting of the protein tau. In AD and in some other dementias, the altered tau can no longer hold the microtubule "tracks" together, causing the transport system to collapse. The tau itself twists into paired helical filaments, like two threads wound around each other. Disruption of the microtubule assembly can lead to cell death. Tau also has long been associated with nerve cell destruction. Although evidence correlates the formation of tangles and the loss of neurons in the part of the brain most affected by AD with increased severity of dementia, until recently there was no evidence that changes in tau protein could directly initiate neuronal degeneration. This changed radically in 1998 when teams of researchers linked several tau mutations on chromosome 17 to inherited dementias characterized by AD-like brain tangles and nerve cell destruction. Now, after years of multinational research on families affected by rare dementias, there is hard evidence that tau can play a primary role in causing at least some cases of neurodegenerative disease. These findings confirmed that mutations in tau alone can lead to dementia. These advances offer new directions for exploring treatments for these dementias, perhaps using drugs that mimic the properties of normal tau or drugs that halt aggregation of tau into brain tangles. Further research is also needed to reassess the relationship between tau and beta-amyloid in dementing disease and to understand how abnormal protein filaments cause cell death in Alzheimer’s disease and other neurodegenerative diseases, including Parkinson’s disease, Huntington’s disease, and prion diseases.
Age-Associated Memory Loss Might Be Reversible. Researchers identified a process by which the normal primate brain degenerates with aging, and were able to show that this degeneration can be reversed by gene therapy. They found that control neurons in a specific area of the brain are most dramatically affected by aging. An actual count of brain cells in rhesus monkeys showed that very few cells are actually lost in the cerebral cortex with advancing age. In contrast, control neurons in another part of the brain (the basal forebrain) were found to shrink in size and to stop making regulatory chemicals, a change that seriously affects the ability to reason and store memories. Using skin cells from each individual monkey, researchers inserted a gene that makes human nerve growth factor (NGF) and then injected the modified cells into the brains of these monkeys. After three months, the brains of the monkeys with the NGF injections had an almost youthful appearance. The number of cells detected was restored to about 92 percent of normal for a young monkey, and the size of the cells was restored to within three percent of normal young values. Such gene transfer approaches to recover cellular function have important implications for the treatment of chronic age-related neurodegenerative disorders, such as AD.
Brain Atrophy Measured by Imaging Techniques Predicts Progression From Mild Cognitive Impairment to AD. Mild cognitive impairment (MCI) is characterized by a memory deficit, but not dementia. Compared to normal memory changes associated with aging, memory loss associated with MCI is more persistent and troublesome. Each year, 12–20 percent of people over age 65 with MCI develop AD, compared with 1–2 percent of people in this same age group without MCI. A recently completed study found that MCI can reliably be clinically defined and diagnosed. The ability to differentiate patients with mild cognitive impairment (MCI) from healthy control subjects and persons with very mild AD hopefully will lead to useful, practical, and cost-effective means to test drug interventions for AD. To help make these distinctions, researchers recently used magnetic resonance imaging (MRI) to determine volume measurements of a region of the brain known as the hippocampus in patients with a clinical diagnosis of MCI. The hippocampus was selected for imaging because this brain structure plays a central role in memory function. Patients were assessed annually for approximately three years using both clinical and cognitive assessments. In older individuals with MCI, the smaller the hippocampus at the beginning of the study, the greater the risk of developing AD later. Imaging studies such as these can actually identify deviations from normal cerebral function or normal anatomy before a clinical diagnosis can be made. This is true for both structural imaging measures such as MRI, and for functional measures such as positron emission tomography (PET). The ability to detect early disease will enable researchers to test the effectiveness of treatments or interventions designed to stop brain changes before clinical deterioration sets in.
Normal Cellular Enzyme Becomes a Marker for AD. Researchers examining the brains of people who had died from AD found abnormally large amounts of a normal enzyme called casein kinase-1 (CK-1) in nerve cells inside cellular sacs (vacuoles) called granulovacuolar degeneration (GVD) bodies. Previous research had shown that these vacuoles tended to accumulate in the hippocampus, a region of the brain important for learning and memory. Looking for an enzyme that adds phosphate to tau molecule, a key protein in the development of dementia, the investigator found a 30-fold increase in one form of CK-1 inside GVD bodies in the hippocampus. This finding enables researchers to use CK-1 as a molecular label for studying the vacuoles and forges a link between them and the plaques and tangles commonly studied in AD brains. Analysis of GVD bodies could provide valuable clues useful both for the diagnosis of AD and for gaining a better understanding of the disease.
Study Results Show Promise for Developing Treatment of Early-Onset AD. Most early-onset AD is the result of mutations in one of two human presenilin genes, PS-1 and PS-2. Mutations in PS-1 are found in about 40% of people with familial (early onset) AD. Every known presenilin mutation affects the processing of amyloid precursor protein (APP) into smaller fragments, such as beta-amyloid peptide, the primary constituent of the distinctive plaques that accumulate in the brains of Alzheimer’s patients. When scientists altered the amino acid sequence of the presenilin protein from its normal sequence in two critical locations, amyloid formation was reduced. Evidence indicates that mutated PS-1 protein may be able to clip the beta-amyloid fragment from APP. If true, the identification of the long-sought enzyme involved in producing neuritic plaques associated with AD should hasten development of drugs that inhibit the enzyme, blocking production of amyloid-beta in much the way cholesterol-lowering drugs work. These studies have implications for the treatment of AD and related disorders of amyloid accumulation. The challenge will be to develop drugs that reduce, but do not eliminate, presenilin since complete elimination of presenilin is lethal in mice, and presenilin is likely to have a similar essential function in humans.
Gene Causing a Form of Familial Dementia May Yield Clues to AD. A form of dementia that spans seven generations of members of the same family in England has been linked to a newly discovered, dominant gene, BRI, on chromosome 13. Familial British dementia (FBD), which has an onset at approximately age 50, is characterized by progressive dementia, muscle spasticity, and loss of muscle tone due to disease of the cerebellum. The predominant pathological lesions are abnormal protein deposits in the brain, plaques in the vicinity of blood vessels, and neurofibrillary tangles. FBD is similar to AD because in both disorders the production of a small insoluble protein is a key feature. Further, the neurofibrillary pathology observed in both FBD and AD is identical. While much remains unknown about the BRI gene and the function of the protein that it produces, understanding how the gene defect causes the disease will lead to insights into the pathogenesis of other neurodegenerative diseases characterized by amyloid "deposition." Understanding how the genetically distinct disorder FBD develops will contribute to efforts to understand the development and progression of the more prevalent Alzheimer’s disease. Further, insights gained in FBD may aid the design and development of treatments intended to disrupt peptide aggregation and prevent the ensuing neurodegeneration not only in FBD and AD but also in other diseases such as those caused by infectious particles call prions.
One Form of the ApoE Gene Protects Brain Cells From Injury. The protein apolipoprotein E (ApoE) participates in the transport of serum lipids (fats) and the redistribution of lipids among cells. Although the mechanism through which it works is unknown, the only accepted risk factor for sporadic late-onset AD is the ApoE4 structural variant of the ApoE gene. To test the hypothesis that ApoE3, but not ApoE4, protects against age-related neurodegeneration, researchers analyzed mice expressing similar levels of human ApoE3 or ApoE4 in the brain. It was determined that ApoE3 protected the brain against excitotoxic injury but that ApoE4 did not. ApoE3, but not ApoE4, also protected against age-dependent neurodegeneration. This study presents compelling evidence to suggest that the presence or absence of a particular ApoE structural variant or isoform affects the way neurons respond to injury. These differences in the effects of ApoE isoforms on neuronal integrity may relate to the increased risk of AD and to the poor outcome after head trauma and stroke in humans. The significance of this finding is that it may help to explain how ApoE4 functions as a risk factor for the development of AD, and, if confirmed, might suggest useful therapeutic strategies that could be started in advance of any cognitive impairment in at-risk individuals.
New Mouse Model Produces Tangles Similar to Those in AD. Developing mouse models with features of human AD is vital in helping researchers gain insights into the etiologies, mechanisms, and progression of AD. Mice implanted with human genes for beta-amyloid, the precursor to neuritic plaques, were developed in 1997. Now, for the first time, researchers have developed a transgenic mouse strain that expresses human tau genes and develops AD-like tau tangles. Unlike their litter-mates that lack the tau gene, these genetically altered mice developed masses of abnormal tau filaments in nerve cells within the spinal cord, cerebral cortex, and brainstem, and in three other critical regions of the central nervous system, as well as undergoing nerve cell degeneration as they aged. While this new strain of transgenic mice does not completely model AD, they closely resemble human diseases that accumulate AD-like tau deposits in the brain. The development of this mouse model will help researchers understand how tau produces disease in the brain, and together with other partial models of AD will move closer to developing effective preventive or treatment interventions against AD.
Study Finds That the Hormone Melatonin Does not Decrease With Age. Melatonin, a natural sleep inducer, is secreted by the pineal gland located deep within the brain. The hormone is produced at high levels during a person’s normal sleeping hours and is lowest during the day. A number of factors, including light and many common medications, such as aspirin, ibuprofen, and beta-blockers, can affect melatonin secretion. In the past two decades, more than 30 reports have suggested that the level of night-time melatonin peak declines progressively with age. These reports have led to a proliferation of over-the-counter supplements aimed at augmenting melatonin levels in the elderly. A five-year study was recently completed that measured serum melatonin levels in 120 healthy men and 24 women aged 18–81. The analysis found no statistically significant difference in night-time melatonin concentrations between the younger and older study participants. This means that in most healthy people, concentration of melatonin probably does not decline with age, and aging probably does not affect the regulation of melatonin secretion.
The Circadian Clock and Aging. Circadian rhythms are pervasive throughout plants and animals, and are closely related to the 24-hour solar day. However, early observations led to the conclusion that regulation of the human circadian clock was different from that of other species, with a median 25.2 hours for a complete cycle. The concept of a shortening of the period with age was used to explain the common observation that many older people go to sleep earlier in the evening and awake earlier in the morning. Recent research using precise measures of naturally occurring melatonin, core body temperature, and cortisol levels in healthy young and older individuals has revealed that the intrinsic period of the human circadian pacemaker averages 24hr 11 min in both age groups. The circadian rhythm is controlled by a small group of neurons deep within the hypothalamus. This "free-running" period is genetically determined and varies very little within individuals of the same species. This counters the belief that the circadian clock speeds up (shortens in duration) as we age, and indicates that the human circadian clock is as stable and precise as that of other animals. This study changes some fundamental assumptions about the causes of sleeplessness among the elderly. Poor sleep is not simply a function of being old. Other factors associated with aging, such as disease, changes in environment, changes in activities, or concurrent age-related processes may contribute to problems of sleep in older persons. The similarity of circadian periods across the animal kingdom suggests that the findings of basic cellular and molecular mechanisms in these model systems will be applicable to solving the problems of sleep and wakefulness in humans.
As life expectancy increases, there is an ever greater need to keep these additional years disease and disability-free. Research has shown that lifestyle and other environmental influences can profoundly impact outcomes of aging, and that remaining healthy and emotionally vital until advanced ages is a realistic expectation. NIA research is helping to define optimal regimens regarding diet, diet supplements, exercise, safety, and other factors to ensure that endurance, strength, and balance are kept at the highest possible level and that the risks of disease and disability are kept to a minimum.
As the 16th century explorer Juan Ponce de Leon discovered, the fountain of youth does not exist. But scientists of the 20th century have discovered that people can feel younger, stay healthier, and experience a better quality of life through exercise, especially in later years. Exercise benefits both the body and mind, with evidence of increased life expectancy, improved mental health, decreased disability, and, most recently, enhanced learning and memory. Despite the proven benefits of exercise, many Americans—especially older Americans—are not engaging in regular, sufficient physical activity. Older persons often are encouraged to slow down or take it easy, and are given little encouragement for engaging in vigorous activity. The challenge for research is to expand our understanding of the benefits of physical fitness, as well as to identify the factors that motivate and deter people from making exercise a part of their daily routine.
Studies have examined the natural history of physical activity and the impact of lifestyle on health outcomes. An early study showed that, controlling for socioeconomic status and health factors, older people who reported no leisure-time physical activity were found to be at 37% increased risk for mortality as compared to those who reported some physical activity. The potency of lack of exercise as a risk factor was confirmed even for those 75 and older. Subsequent findings made it clear that older people are not exercising as much as they should to achieve the health benefits possible through regular physical activity. Data from the 1988–1991 National Health and Nutrition Examination Study illustrated how inactivity rates increase as people age and differ between men and women, and among racial and ethnic groups. Women were found to be considerably less active than men, and prevalence of leisure-time inactivity was higher among African-Americans and Mexican-Americans than among Caucasians.
The good news is that even moderate physical activity can reap important health benefits, and that it is never too late to start adopting healthier lifestyles. In a recent study, physically active older individuals had double the likelihood of living the remainder of their lives with no disability compared to sedentary adults. They are also more likely to live to advanced old age and to remain independent in basic self-care activities in the year prior to their deaths. In this study, moderate physical activity included walking and gardening, activities feasible for many older adults. Groundbreaking studies reported in 1994 involving frail nursing home residents from 72 to 98 years old found that a 10-week resistance exercise program approximately doubled leg strength, increased walking speed, improved stair-climbing power, and led to increased spontaneous physical activity, when compared to controls.
Since the early 1990s, several exercise-oriented intervention studies have been conducted to reduce frailty and injuries. Falls are the primary cause of the more than 250,000 hip fractures that occur each year among older persons. One intervention strategy that included exercise produced a 44% lower rate of falls than a control group. Exercise can also benefit people suffering from a variety of physical ailments, such as osteoarthritis, a common condition that causes pain and activity limitation in older people. For example, the Fitness Arthritis and Seniors Trial tested the long-term utility of aerobic training (walking) or resistance training (weight lifting) in helping older people with knee osteoarthritis maintain their function and quality of life. Participants reported less pain and better function than controls. Studies on the effects of exercise on chronic pain and peripheral arterial disease observed similar positive results, particularly regarding improved pain management.
Studies have begun to identify a link between exercise and increased life expectancy. In one study, higher fitness was associated with lower mortality rates in men aged 20 to 82. The study found that unfit men age 60 and over who later became fit had death rates 50% lower than those who remained unfit. In another study, people who reported moderate to high levels of exercise lived three or more years longer than less active study participants.
Research on the effects of exercise on the body’s neurological function have produced exciting new findings. Animal studies have shown that exercise can enhance generation of brain cells, which may someday mean that replacement of neurons lost through age, trauma, or disease might be enhanced via a regimen that includes the use of exercise. In humans, exercise can attenuate age-related decline in some cognitive skills. The beneficial effect of aerobic exercise is selective—it seems to affect only those functions associated with frontal regions of the brain. A recent study examined the effect of increased light aerobic activity on tests of planning, scheduling, and short-term memory, and the inhibition of inappropriate responses. Remarkably, previously sedentary adults, age 60 to 75, with small increases in aerobic fitness due to six months of light to moderate walking, showed substantial improvement in these higher thinking tasks. There were no increases in tasks associated with other regions of the brain, such as short-term memory.
Exercise not only helps daytime functioning, it also helps individuals get a good night’s sleep. Two independent randomized controlled trials among older adults with moderate sleep complaints showed that exercise improved quality of sleep. The first study used an intervention of low-impact aerobics and brisk walking for 30 to 40 minutes four times a week over a 16-week period. Compared to a control group, who did not change their physical activity levels, the exercise group had significant improvements in sleep latency (a 50% reduction, about 15 minutes), sleep duration (increase of almost an hour), and quality of sleep.
The second study used an intervention of progressive resistance training of the large muscle groups for about one hour three days a week for 10 weeks. The control group participated in a health-education program. The participants were depressed elders, aged 60 to 84 years. The exercise regimen resulted in subjective improvement of sleep quality as well as a 50% reduction in depression measures. Work continues on which types of exercise activities and intervention strategies are most effective for initiating and maintaining physical activity within a diverse, aging population.
Delirium Can Be Prevented in Hospitalized Older Patients. Delirium, an acute confusional state, in older hospitalized older patients is associated with poor outcomes, and is a common, serious, and potentially preventable source of both prolonged illness and early death. Between 20–30 percent of all hospitalized elderly patients have episodes of delirium, resulting in treatment costs exceeding $4 billion per year in the U.S.(5) Previous studies of delirium focused on the treatment of delirium rather than on primary prevention. A recent study evaluated the effectiveness of a multicomponent strategy for the prevention of delirium. Study participants received either usual, standard hospital care or care under a multidisciplinary team of specialists that included staff nurses, recreational therapists, physical therapists, geriatricians, and trained volunteers. Patients in this study had one or more of six risk factors for delirium, including cognitive impairment, sleep deprivation, immobility, dehydration, or impaired vision or hearing. To address these risk factors, team members were trained to recognize and counteract the danger signs before confusion, agitation, and hallucinations set in. Interventions include making sure patients got enough fluids, taking them for walks, and providing warm drinks at bedtime to promote sleep. While 15% of patients receiving standard hospital services experienced at least one episode of delirium, only 9.9% of those receiving the team approach experienced an episode. Once an initial episode of delirium had occurred, however, the intervention had no significant effect on the severity of delirium or the likelihood of recurrence. This study holds substantial promise for the prevention of delirium in hospitalized older patients. Further evaluation is needed to determine the cost effectiveness of intervention to prevent delirium and its effects on related outcomes, such as mortality, rehospitalization, institutionalization, use of home health care, and long-term cognitive functioning.
Predictors of Healthy Aging Can Be Identified and Interventions Can Reduce Risk of Disability. There is a need to understand whether there are modifiable risk factors that can decrease the risk of disability and death with aging. A long-term study with Japanese-American men in Hawaii has shown that these men have one of the highest life expectancies of all Americans. Because a number of baseline measurements were taken of these men in midlife, from 45 to 68, it was possible to explore predictors of long life expectancy and prevention of physical disability. Among over 6500 healthy men at baseline, about 60% remained free of major illness and were not physically or cognitively impaired over the next 25 years. Data from midlife that proved to be predictive of healthy aging included optimal blood pressure, low blood sugar and cholesterol levels, lack of obesity, lack of smoking, and strong hand grip. At an older age the men were examined to determine the presence of functional limitation and disability. Of various factors considered, midlife hand grip strength was associated with less physical disability and faster walking speed. In a clinical trial, participants were randomized into intervention and control groups. At the end of one year after a regimen of increased physical activity and chronic-illness self-management, the intervention group experienced fewer hospitalizations and fewer total hospital days. Factors leading to a long and active life are of prime importance as the population ages worldwide. This study suggests that preventive and/or therapeutic interventions are most effective when initiated at younger ages, although the clinical trial results suggest that successful intervention can occur at older ages. Researchers will need to work with clinicians to develop strategies to address modifiable risk factors in order to better promote healthy aging.
Testosterone Replacement for Older Men May Have Protective Effects Against Age-Related Diseases. Many older men have blood levels of testosterone well below the normal range for younger men. Earlier studies have shown that low testosterone levels may increase risk factors for disease and disability, including loss of bone (leading to osteoporosis and fractures), loss of muscle (causing decreased strength), and increases in body fat (increasing risks for diabetes and heart disease). In a recently completed clinical trial of men over 65 years old with low serum testosterone, study participants were given a testosterone or placebo skin patch for three years. Levels of testosterone in the treatment group rose to those generally found in younger men. Men with the lowest endogenous serum testosterone (3 micrograms per liter or less) prior to beginning the trial had significant increases in bone density in response to testosterone replacement. The testosterone treatment also increased lean body tissue and significantly decreased body fat. Study participants were monitored for possible adverse treatment effects, particularly on the prostate. Testosterone treatment did not increase symptoms of an enlarged prostate, such as impaired urinary function, nor was there statistically significant evidence that the administered testosterone increased the incidence of prostate cancer. The results of this study suggest that testosterone replacement could help protect many older men with low testosterone levels against common diseases of aging such as diabetes, heart disease, and osteoporosis. However the possibility that testosterone replacement could increase adverse events such as prostate diseases, though not observed in this small study, reinforces the need for well-designed larger studies as well as the development of strategies to minimize risks of testosterone therapy while still providing benefits.
Postmenopausal Estrogen Has a Positive Influence on Women’s Arteries. Arterial stiffness has been identified as a potential risk factor for cardiovascular disease. Earlier research has shown that estrogen may improve blood vessel pliability by altering the structure and function of vascular tissue, including smooth muscle cells. This study examined the influence of age and current estrogen replacement therapy (ERT) on stiffness in the common carotid arteries (the main arteries that pass up the neck and supply blood to the head). The common carotid arteries of 172 women, 37 of whom were current users of ERT, were examined by ultrasound, and the degree of arterial stiffness was measured. Arterial stiffness was found to increase linearly with age, and was modestly related to other cardiac risk factors. The degree of stiffness was lower in women using ERT than in postmenopausal nonusers. Furthermore, the effects of age and ERT on the stiffness persisted after adjustments for other cardiovascular risk factors. Carotid stiffness was similar in ERT users, whether or not they also took progesterone. This study suggests that the cardiovascular protection seen in women using ERT may involve overall reduction of age-associated arterial stiffening.
Chronic Inflammation in the Elderly Predicts Disability and Early Death. Inflammation is a normal biologic response of the immune system to a number of different stimuli, including infections, allergens, and physical trauma. However, inflammation can become chronic and increase the onset and severity of a number of age-related disabilities and diseases. An indicator of this process is the elevation of a proinflammatory protein, interleukin-6 (IL-6), which plays a central role in inflammation and increases with age. High circulating levels of IL-6 may contribute to functional decline in old age and an increase is observed in such diverse conditions as depression, heart failure, and arthritis. One study of nearly 1,700 men and women, aged 70 or greater living in North Carolina, measured IL-6 levels against a standardized test for depression. After controlling for age, race, and gender, IL-6 levels remained the only biologic variable significantly associated with depression. In another study in men and women 71 years or older, participants with the highest levels of interleukin-6 were almost twice as likely to develop mobility-disability and were about twice as likely to die within 5 years of the beginning of the study. It is known that IL-6 stimulates the synthesis of C-reactive protein, an indicator of systemic inflammation. When levels of both IL-6 and C-reactive protein were elevated simultaneously, there was a 3-fold increased risk of mortality. Further studies are needed to improve our understanding of the complicated system of stimulus and response with regard to inflammation. These findings may broaden our understanding of the health correlates and consequences of chronic inflammation, as well as provide a new way to identify high-risk individuals to determine whether they would benefit from anti-inflammatory intervention.
Behavioral Training Is More Effective Than Drug Therapy for Urge Urinary Incontinence. Approximately 15 million Americans adults have urinary incontinence (UI) with associated health costs estimated in a range of $16–$26 billion dollars annually.(6),(7) Urinary incontinence is especially a problem for women. Nearly 40 percent of community dwelling women age 60 years and older suffer from some form of UI. While behavioral training and drug therapy have both been previously demonstrated to be effective treatments for urge urinary incontinence in older adults, drug therapy is commonly used as the first course of treatment. A recent clinical trial directly compared behavioral training (instrument-assisted pelvic muscle exercises to improve bladder control) to drug treatment for urge UI in older women and demonstrated that behavioral training was significantly more effective than drug therapy in reducing the episodes of accidental urine loss. Thus, behavioral training should be considered the first treatment option given the potential side effects of drug therapy, and to avoid further problems with drug interactions among older persons taking multiple medications.
Research on the biology of aging has led to a revolution in understanding the cellular and molecular changes that occur with aging. This new gerontology investigates the progressive biological and physiological changes that normally occur with advancing age and the abnormal changes that are risk factors for or accompany age-related disease states. Progress is being made in understanding the gradual changes in structure and function that occur in the brain and nerves, bone and muscle, heart and blood vessels, hormones, nutritional processes, immune responses, and other aspects of the body. Research has begun to reveal the biologic factors associated with extended longevity in humans and animal models. The ultimate goal of this effort is to develop interventions to reduce or delay age-related degenerative processes in humans.
Mitochondrial DNA Mutations Increase With Aging. One hypothesis of the cause of aging is the accumulation of mutations in mitochondrial DNA (mtDNA). Although earlier research has shown that a particular deletion mutation of mitochondrial DNA increases with age, it appeared that this mutation only occurred in less than 4 percent of mtDNA molecules. However the methods used to quantitate the level of this mutation would not have detected other deletions, so it was argued by some that the common deletion mutation represented the "tip of the iceberg" of mitochondrial mutations. Skeptics responded that this unproven hypothesis represented wishful thinking. By use of a sensitive method to look at point mutations in mitochondrial DNA, researchers found hard evidence that mtDNA point mutations increase with aging and mitochondria deteriorate as people age. These scientists show that one particular point mutation in the control region of the mtDNA occurs in a high proportion of the mtDNA molecules of more than 50% of people over the age of 65, but is absent in younger individuals. Because the mitochondria are the cellular sites for energy metabolism, deterioration of mitochondria could deprive cells of the energy they need to function and ultimately could lead to premature cell death.
Caloric Restriction Prevents Age-Associated Changes in Gene Expression. Most multicellular organisms exhibit a progressive and irreversible physiologic decline during the aging process. The only intervention known to slow the intrinsic rate of aging in mammals is caloric restriction. Given 30 to 40 percent fewer calories than in usual feeding schedules, but fed all the necessary nutrients, rodents and other nonprimate laboratory animals studied not only have lived far beyond their normal lifespans but have reduced rates of several diseases, especially cancers. In a new study, the gene expression profile of the aging process was analyzed in skeletal muscle of mice. Of the 6347 genes surveyed by new microarray techniques, only 58 (0.9%) displayed a greater than twofold decrease in expression. Thus, the aging process is unlikely to be due to large, widespread alterations in gene expression. The major effect of caloric restriction seems to be to heighten animals’ stress response in response to damage to proteins and other large molecules. Caloric restriction also completely or partially suppressed age-associated alterations in expression of a large proportion of genes. This is the first global assessment of the aging process in mammals at the molecular level. Potentially, gene expression profiles can be used to assess the biological age of mammalian tissues, providing a tool to evaluate experimental interventions.
Link Established Between Telomeres and Mammalian Aging. Telomeres are highly repetitive DNA sequences located at the end of chromosomes. They are essential for the stability of chromosomes and cell survival in a wide variety of organisms. In human cells grown in culture, telomere length shortens with each cell division and the progressive telomere shortening ultimately limits the ability of cells to divide. To test the possibility of a link between telomere shortening and aging of an organism, investigators have created genetically altered mice lacking telomerase, an enzyme that adds new telomeric DNA sequences to existing telomeres. In this transgenic model, telomeres progressively shortened throughout the lifespan, providing a unique opportunity to understand the cellular consequences and aging significance of telomere shortening in the living animal. Although loss of telomeres did not elicit a full spectrum of the classical symptoms of aging, age-dependent telomere shortening was associated with a shortened lifespan, reduced capacity to respond to physiological stress, slow wound healing, and an increased incidence of spontaneous cancers. As individuals age, aged organs show a markedly diminished capacity to cope with acute and chronic stress. The telomerase-deficient mouse provides a valuable model to study the role of telomere maintenance in cellular stress responses in the aging organism.
A goal of NIA behavioral and social research is to maintain or enhance the health and well-being, including physical and cognitive function, of older individuals throughout the lifespan. For example, new interventions are being developed to encourage long-term changes in health behaviors that will lead to reduced risk of disease and disability. Cognitive interventions are being tested to maintain cognitive function and retain independence. Components of the physical environment are being redesigned to match the skills and abilities of older persons, thus helping to prevent injuries and to improve performance of daily activities. Such human factors research has produced new and improved medical devices and treatment regimens, instructional designs, and product labeling. As more older people are able and willing to work well into late adulthood, researchers are studying the physical and social barriers to their sustained participation in the workforce and the factors needed to enhance their skills and productivity. A related body of demographic research documents trends in health, retirement, long-term care, and the economic aspects of aging, and uncovers their causes and interrelationships.
Social and Productive Activities Confer Survival Advantages to the Elderly. When previous studies found that older people who remained active lived longer, scientists assumed that the survival advantage resulted from improved cardiopulmonary fitness attributable to physical activity. A new study suggests that social activities (church attendance, travel, etc.) and productive activities (gardening, community work, etc.) involving little or no enhancement of fitness lowered the risk of all-cause mortality over a 13-year period to a degree similar to that achieved by fitness activities (e.g., swimming, and walking). This study suggests that a wider range of mechanisms, both psychological and psychosocial, may be involved in the association between activity and mortality than had been previously thought. This finding has important implications for public policy and clinical practice. If confirmed, it suggests that clinicians might consider recommending a broader range of activity options for older patients.
Centenarians Live Most of Their Lives in Good Health. Scientists have found preliminary evidence that many centenarians remain functionally independent for the vast majority of their lives and then experience a relative rapid decline near the end of their lives. Relative to others in the older population, they also appear to either experience a marked delay in the onset or, in some cases, escape diseases such as cancer and Alzheimer’s disease. Scientists also find a strong familial component to extreme longevity. Siblings of centenarians tend to live longer compared to siblings of individuals who died in their mid-70’s. This may be due in part to shared genetic traits among family members. Understanding the genetic and environmental factors responsible for centenarians’ prolonged good health could provide insights for improving the health of all older people. Further work is needed to elucidate the genetic and environmental factors that contribute to centenarians’ extreme longevity.
Socioeconomic Status and Health Disparities Are Strongly Related Over the Life Course. There is a striking and well-documented relationship between socioeconomic status, health, and longevity. People with higher incomes and more wealth tend to be healthier and to live longer. The causes of this relationship are largely unknown, but have been assumed to be related to health behaviors and access to care. In a recent study, African-American men were found to have lower life expectancy in disparate income groups than did white men in the same income groups for the years 1979 to 1989. African-American men with family incomes below $10,000 averaged 7.4 fewer years of life than black men in families with more than $25,000; among white men, the differential between the two income groups was 6.6 years. Less work has been focused on the effect of health events on subsequent income and wealth. The strong interrelationship between health and wealth at older ages may be due, in large part, to the adverse economic impact of major health events. One major reduction in wealth appears to be reduced earnings that stem from taking early retirement or otherwise decreasing work. People who have heart attacks, strokes, or other acute health events are especially likely to reduce their work levels. There are equally large reductions in wealth among those with and without health insurance (although those with health insurance have lower out-of-pocket medical expenses), suggesting that health insurance does not fully protect people from the economic costs of major illnesses. This finding demonstrates how differences in health status can cause differences in economic circumstances. These results also suggest some direction for policy. They show, for example, that health insurance deals with only a small part of the economic cost of declining health. The much larger economic costs of decreased work and lost earnings might be more effectively addressed in other ways. To aid in understanding this causal relation between health and wealth, future clinical trials could include more economic content so that the impacts of health on economic status can be measured.
Neighborhood and Socioeconomic Characteristics Hamper Progress in Fitness. Physical inactivity is a leading cause of both death and disability among older adults. Recent analyses from the Alameda County Study show that socioeconomic variables such as neighborhood characteristics affect physical activity levels and thus may contribute to health disparities. Living in a poor neighborhood is associated with a decline in physical activity, even adjusting for age, individual income, education, smoking status, body mass index, and alcohol consumption. Other survey analyses reveal that poor weather and fear of crime were majors barriers to exercise among low income urban older adults, as was the lack of information from physicians and family/friends regarding the safety and benefits of exercise. These studies demonstrate the importance of designing physical activity/exercise programs that can counter the negative effects of disadvantaged social conditions.
Preventing Alzheimer’s Disease: The AD Prevention Initiative. NIA is accelerating efforts to develop novel compounds for treating the cognitive impairment and behavioral symptoms associated with AD. Leads for promising strategies are being identified through a focused effort to understand and predict the initial stages and events in the brain that lead over several decades to AD development. Research has brought science closer to the threshold of discovering effective agents that retard deposition of brain plaques in animal models. As leads are identified and developed in the test tube and in experimental animals, the new findings will be will be translated into clinical interventions. The translation process will involve testing of drugs that target crucial pathways as well as incorporation of efficient processes for channeling drugs of interest into appropriately designed clinical trials. Plans for clinical trials will increasingly emphasize AD prevention, including trials in persons with normal cognition. The first NIH prevention trial for AD, comparing the effects of vitamin E and Aricept, was recently begun at more than 70 sites in persons diagnosed with mild cognitive impairment. The goal of this trial is to prevent the development of AD symptoms in these individuals, who are at high risk for developing AD. Upcoming trials will examine the effectiveness of ibuprofen (an anti-inflammatory drug) in reducing the risk of AD, the effect of estrogen replacement therapy in preventing AD in women with a family history of the disease, and whether treatment with a variety of agents, such as aspirin, vitamin E, antioxidants, or combined folate/B6/B12 supplementation can prevent older women from developing AD. Several approaches will be initiated to increase the efficiency and cost-effectiveness of developing new and novel approaches to AD drug discovery, development, and testing. Future initiatives will also develop more effective methods to treat and manage behavioral symptoms in persons who have AD and to significantly reduce caregiver burdens. Studies will be launched to develop and test new ways of managing the daily activities and stresses of caring for people with AD as well as to help prevent hospitalizations, delay nursing home admission, and prevent caregiver burden.
Combating Health Disparities in AD and Other Neurodegenerative Diseases. It is estimated that the percentage of racial minorities and Hispanics in the population of Americans over the age of 85 will increase from 13% in the year 2000 to more than 30% in 2050.(8) It is thus increasingly urgent to identify genetic and nongenetic risk and protective factors for AD and other neurodegenerative diseases of aging in racially and ethnically diverse populations. These risk factors could vary with race, ethnicity, gender and socioeconomic status. Studies of the relative risk of AD for African Americans and Hispanics compared to other ethnic groups have yielded inconsistent findings, indicating the need for further research in this area. Differences in AD risk may exist among ethnic groups in prevalence and incidence rates and in the relative importance of particular genetic and nongenetic risk factors. For example, the increased risk for developing AD conferred by carrying one ApoE4 allele is greatest for Japanese, intermediate in Caucasians, but is not evident in African Americans. The NIA is stimulating research to assess and compare prevalence and incidence rates among different ethnic subgroups, using culturally appropriate instruments; to determine the importance of particular genetic risk and protective factors as well as potential nongenetic risk factors, including comorbid conditions such as cardiovascular and cerebrovascular disease; to determine the risk of developing AD after an initial diagnosis of mild cognitive impairment; and to identify lifestyle differences conferring risk or protection, factors such as early development, diet and education.
Maintaining a Healthy Brain. Neurons atrophy and degenerate throughout life in response to many influences during "normal" development as well as from emotional stress and other pathological processes. Disturbances of cognition and emotion afflict millions of people at every stage of the lifespan. Yet we should not accept nervous system decline, any more than we would now accept deteriorating cardiovascular health, as the inevitable consequence of disease or of normal aging. We must learn how to prevent or reverse the loss of brain function through early detection and intervention. The Healthy Brain Initiative will develop plans for intervention to improve cognitive and emotional health of the American public. The NIA, National Institute of Mental Health, and National Institute of Neurological Disorder and Stroke will organize the initial phase of the study, with other interested NIH institutes or centers invited to participate.
Reducing Health Disparities. Although an array of factors has been associated with health disparities—including race, ethnicity, gender, socioeconomic status, age, education, and occupation—an intense research effort is needed to identify the causes of these differences. Recent research has found that modification of the Mini-Mental State Examination test by controlling for education and gender resulted in the elimination of significant differences in imputed mental impairment between older Hispanics and non-Hispanic whites. In addition, certain chronic diseases, such as diabetes, hypertension, stroke, and cardiovascular disease, tend to be more prevalent in several racial and ethnic groups. New NIA studies will focus on the influence on aging health of early and midlife health, nutrition, education, and health care. Research will also expand understanding of how to prevent or lessen the effects of disease by designing more culturally appropriate interventions and modes of health information dissemination and by discovering means to enhance healthy behaviors in older racial and ethnic populations.
Assessing the Impact of Aging on Cardiovascular Disease. Diseases of the heart and blood vessels are the leading cause of hospitalization and death in older Americans. The NIA is pursuing a broad program of basic and clinical cardiovascular research, often in collaboration with the National Heart, Lung, and Blood Institute. Recent findings have demonstrated the effectiveness of both pharmacologic and lifestyle approaches in reducing hypertension and preventing heart disease and stroke. Characterization of age-associated changes in both the structure and function of the heart and blood vessels is vital to the development of newer, more effective treatment and prevention interventions. Research priorities include identifying genetic and environmental risk factors for hypertension, heart disease, and stroke. Studies are ongoing to determine the causes of age-associated increases in vascular stiffness, a potential risk factor for cardiovascular disease. Other research will focus on age-related changes in the structure and function of the heart’s conduction system that can increase the risk of cardiac arrhythmias, especially atrial fibrillation that if uncorrected can lead to strokes. Additional priorities include determining the reasons for gender and racial differences in the aging cardiovascular system, delineating the relationship of cardiac enlargement to aging and disease development, and reducing the progression of early atherosclerotic disease.
Treating and Reducing the Risk of Cancer. The second leading cause of death among the elderly is cancer, with individuals age 65 and over accounting for 70 percent of cancer mortality in the U.S.(9) In collaboration with the National Cancer Institute (NCI), the NIA is expanding basic and clinical research on breast, prostate, and colon cancers, common in older people, and launching a new initiative to expand participation of older cancer patients in clinical trials. This research focuses on age-related changes that contribute to increased cancer incidence and mortality in older persons, aggressive tumor behavior in the aged patient, and the impact of previous or concurrent conditions and disabilities on the cancer experience of older patients. Specific research topics include: dose adjustment for antitumor agents and radiation therapy, diagnostic cancer imaging, how coexisting diseases affect cancer treatment and survival outcome, and survival advantages or disadvantages of minority or ethnic populations.
Enhancing Musculoskeletal Function. Osteoporosis, osteoarthritis, and age-related loss of muscle mass (or sarcopenia) contribute to frailty and injury in millions of older people. The NIA supports several initiatives to unravel the underlying mechanisms of aging in bone, muscle, and joints, and to design and evaluate effective prevention and intervention strategies for age-related musculoskeletal decline. For example, factors are being explored to define the influences that can predispose older people to fractures and to develop effective prevention and intervention strategies for age-related musculoskeletal decline. The NIA is collaborating with the NIAMS to expedite the development and evaluation of novel therapies for osteoarthritis. The initiative is designed to stimulate innovative strategies to evaluate the process of joint destruction and to accelerate findings in bone and cartilage turnover and genetics related to osteoarthritis.
Evaluating Hormone Replacement Therapy and Dietary Supplements. Counteracting the effects of aging by supplementing hormones—such as estrogen, testosterone, human growth hormone, melatonin, and DHEA (dehydroepiandrosterone)—is an area of active study, but there are concerns that individuals may be taking such agents before their safety and efficacy have been fully assessed. Although levels of some of these hormones may decline, on average, as people age, maintaining levels that are normal at younger ages may not be needed, or even desirable, as a person grows older. Even if effective, supplementation may entail risks. More research is needed to determine how the biologic action of these hormones changes in older people and to assess whether replacement of these hormones will improve health. Based on recent results, planning has begun for a trial to test the impact of testosterone replacement in older men with low testosterone on the incidence of fractures and other potentially important outcomes, such as serum lipids, cardiovascular events, and potential adverse effects on the prostate. Also needed are alternative approaches to realize the benefits of estrogen, testosterone, and other hormonal therapies while minimizing risks and undesirable side-effects. Two principal strategies being pursued. One involves synthesizing compounds that produce the beneficial responses of hormones in the body without detrimental side effects. The other would increase or decrease hormone production in specific body tissues to achieve levels favorable to health. When successful, these innovative approaches will, for example, enable men and women to benefit from the properties of estrogen without estrogen’s unwanted side effects. Related research is underway on the ability of antioxidants such as vitamins C and E to prevent cancer, delay aging, or keep cognition intact. Antioxidants are found in common foods and act as scavengers for oxygen radicals, molecules generated when cells produce energy, that can cause long-term harm and degradation to the body. NIA research will address the special dietary and nutrient needs of elderly persons, especially nutrients capable of delaying or mitigating the degenerative diseases that often accompany aging.
Understanding the Disability Decline and Its Implications. Recent findings have revealed dramatic and unexpected reductions in rates of disability among older persons compared to projected levels. Studies have shown that disability levels for people age 65 and older have been falling at an accelerating pace since 1982, and that the benefits of this trend extend both to men and women and to minority groups. This decline contributes to improved functional ability for individuals and could have important economic and social implications. Continuing the current pace of disability decline over the next 50 years could prevent increases in the number of disabled Americans that will otherwise occur in the face of the demographic challenge posed by the baby boom and overall population aging. In order to sustain continued decreases in disability, it will be critical to identify the underlying causes of the disability decline. Studies have identified social, educational, public health, and biomedical variables that affect rates of disability. Further research will define trends likely to extend the disability decline, such as improvements in health-related behaviors, the increasing education levels of older people, improvements in the availability and effectiveness of assistive devices, disease prevention, and improvements in the treatments of conditions that lead to disability. Based on the explanatory factors identified in this study, additional research will consider the specific interventions, behavioral changes, and survival attributes that could most effectively enhance the disability decline. Efforts will also be made to improve projections of disease and disability rates and to clarify the implications of the disability decline for changes in family demography and medical care costs.
Understanding the Genetic Basis of Aging, Longevity, Age-Related Diseases, and Behavior. Interactions between genetic and environmental factors are major determinants of aging and longevity in a many species, including humans. NIA studies have begun to reveal the biologic factors associated with extended longevity in humans and animal models, implicating numerous genes in normal aging processes, age-related pathologies and diseases, and longevity. Some of these genes are associated with dramatic extension of lifespan. Using advanced technology, the NIA plans to accelerate its efforts to discover additional age- and longevity-related genes and to characterize their biological function. A new initiative will extend studies of longevity-associated genes, changes in gene expression patterns, and the genetic epidemiology of human longevity. The ultimate goal of this effort is to develop interventions to reduce or delay age-related degenerative processes in humans. In addition, revolutionary advances in the fields of quantitative and molecular genetics hold great promise in the search for the genetic determinants of complex behaviors. Studies in humans can help identify the relative contributions of environment and heritability to dementia, cognitive abilities, physical functioning, well-being, and social aging. New techniques can track the developmental course of genetic contributions to behavior, identify genetic heterogeneity, and explore genetic links between the normal and abnormal.
Understanding the Effect of Caloric Restriction on Aging. Caloric restriction (CR) has long been known to extend lifespan markedly in rodents and other laboratory animals studied and to delay in these species the onset of numerous age-related diseases common in humans. Over the last two decades, CR has also been shown to delay a wide variety of aging-related changes, including specific cellular and molecular alterations. Most recently, researchers have identified changes in physiologic function in calorically restricted rhesus monkeys that suggest delays in aging-related decline in these primates. These results could have important implications for human intervention programs. Animal studies are now planned to determine the effects on aging of interventions that mimic metabolic effects of CR. Also being considered are preliminary human intervention studies designed to determine whether CR and physical activity differ in their long-term effects on obesity, body composition, prevention and susceptibility to age-related diseases.
Exploring the Potential of Stem Cells and Cell Replacement in Aging. Stem cells in human tissues retain the capacity for self-renewal and the potential to become many of the cell types in the human body. This capacity holds enormous potential for cell replacement or tissue repair therapy in many degenerative diseases of aging, including Alzheimer’s disease, Parkinson’s disease, stroke, myocardial infarction, musculoskeletal disorders, immune system dysfunction, and diabetes. Research is needed to explore the role of stem cells in repairing tissue damage and recovering organ function as organisms age. Emerging research findings suggest that it may be possible to harness the multipotential nature of stem cells to maintain tissue structure and function in aging. Much remains to be learned, however, about the basic biology of stem cells before effective cell therapy can be realized. The NIA is developing an initiative on stem cells in aging that will complement as well as encourage collaboration in activities of other NIH components.
Monitoring Health Through Demography. As the world’s older population grows, demographic research enables us to monitor the impact of population aging on the global burden of chronic disease and disability. This knowledge enables us to identify health and economic trends and to recognize opportunities for research on their causes and impact. NIA will collaborate with other NIH institutes in studying the changes in health and functional status over time of disabled and chronically ill older people. Research is being developed to improve data on burdens and costs of diseases. In response to advances suggesting that disability rates of older Americans are declining, researchers are developing studies to identify and quantify the specific underlying causes contributing to the decline, as well as to design interventions. Demographic research is also planned to track the dynamics underlying the increase in old-age life expectancy in the U.S. and to define the implications of changes in health, disability, and life expectancy for national policies on retirement and on programs for the elderly. A special focus is being developed to provide the necessary data for understanding the large variations in health across racial and ethnic populations.
Preventing Medication Misuse in Aging Populations. Older people are especially vulnerable to medication-related problems, not only because of the large numbers of medications taken for multiple health problems, but also because of both physiological and functional changes with aging. With an increased number of new drugs in development to combat the diseases and chronic conditions associated with aging, there are many more opportunities for medication misuse resulting either from patient noncompliance with medical regimens or physician prescribing errors. The NIA is planning new studies as part of a multifaceted research agenda for understanding and alleviating medication problems in older populations. Reducing these medication problems will help enhance the health and quality of older people and also save unnecessary health care expenditures.
Gathering Data on Elder Abuse and Neglect. The Institute of Medicine (IOM) reported that there was a "paucity of research" on elder abuse and neglect. A recent NIA-supported study showed that, compared to nonabused elders, victims of abuse have three times higher mortality from all causes, and those identified as self-neglected have almost twice the mortality. Supporting the need for systematic study of elder abuse and neglect are the lack of reliable, probability-based national prevalence estimates of elder abuse; the growing numbers of older people; the increasing public awareness of the problem; legal requirements for reporting; recent advances in techniques to elicit both the reporting and estimation of socially stigmatized behaviors; and the availability of interventions that facilitate prevention and treatment programs. The NIA is developing plans to mount a study on elder abuse in collaboration with the National Academy of Science and relevant federal agencies. This initiative will employ racially and ethnically sensitive measurement techniques for elder abuse and neglect that are applicable to people of both genders and of all socioeconomic statuses.
Providing the Science Base for Caregiving Policies and Programs. Caregiving and long-term care are becoming major national policy issues. In 1997, at least one family member in each of more than 22 million households in the U.S. was providing some form of unpaid caregiving. Caregiving often is burdensome and stressful, and can lead to major emotional trauma or illness for the caregiver. While the number of older persons is expected to increase rapidly, demographic changes in families (more childless, one-child, and step-families) and increasing participation of women in the work force suggest a likely decrease in provision of informal care for aging baby boomers. Studies using new data sources are being planned to provide the information we need to carefully examine caregiving needs, patterns of family caregiving, decisionmaking on providing care, and costs of care. For example, the 1999 National Long-term Care Survey caregiver supplement will provide new trend data on caregivers’ health and functional status, the types and amounts of care delivered, the implications of new family roles and relationships, and the links between family and formal care. New community surveys, such as the Washington University Black Rural and Urban Caregiver Study, will promote research on how race, ethnicity, and socioeconomic status affect caregiving needs and related issues. Initiatives will also examine the effectiveness of various interventions aimed at reducing Alzheimer’s disease care burdens, such as skills training and environmental modifications, with special attention to caregiving in minority populations.
Developing and Distributing Research Resources. Physical resources—such as animal models, chemicals, tools, and other technologies--play a critical role in research. The NIA develops and distributes these high quality resources to investigators efficiently and at reduced cost. These resources include:
-
Central aging colonies of animal models, including genetically altered animals, necessary for research on aging processes and specific age-related diseases
-
Cell cultures and tissue, cell, and blood banks for basic and epidemiologic research
-
DNA resources for genetics
-
Imaging technologies for exploring the body, from the interior of the cell to organ systems
-
Bioinformatics technologies to record and analyze findings on basic biological research
The NIA will continue to identify and evaluate opportunities for providing research resources and infrastructure development using the advice of extramural and intramural researchers. The NIA is also evolving information technologies to assure broad access to archived data vital to researchers and policymakers and to ensure protection of anonymity and confidentiality of participants in clinical studies. In conjunction with other NIH institutes, the NIA will support research on new mathematical and informatics methodologies and on improved instrumentation and computational techniques for modeling systems changes in aging.
Throughout the world, populations are aging at an unprecedented rate. There is an urgent need to maintain the highest degree of function and quality of life for the longest period of time in older people. An understanding of aging and its relationship to disease and disability is one of the surest means to gain the knowledge needed to achieve these goals. Aging research has made significant strides in revealing the underlying processes that help determine longevity and the risk of disease. We are thus learning about factors important to maintain or improve high physical function and reduce premature death. We are developing tools to improve not only strength and balance but cognitive function, including memory, in old age. These advances are fueling health promotion and an optimism in attaining a successful old age. Efforts are intensifying to conquer Alzheimer’s disease and to prevent or delay other age-related diseases and disorders. All of us stand to gain from the prospect of a healthy, fully engaged older population: these include the rapidly growing numbers of the aged, those they care for and who care for them, and all who will join these groups in the future.
The Fiscal Year 2001 budget request for the NIA is $721,651,000, excluding AIDS, an increase of $37,933,000 and 5.5 percent over the FY 2000 level. Included in this total is $23,500,000 for the following NIH Areas of Special Emphasis:
| Biology of Brain Disorders |
$9,000,000 |
| New Approaches to Pathogenesis |
1,000,000 |
| New Preventive Strategies Against Disease |
5,000,000 |
| New Avenues for the Development of Therapeutics |
3,000,000 |
| Genetic Medicine |
2,000,000 |
| Health Disparities |
3,500,000 |
A five year history of FTEs and Funding Levels for the NIA are shown in the graphs below:
One of NIH's highest priorities is the funding of medical research through research project grants (RPGs). Support for RPGs allows NIH to sustain the scientific momentum of investigator-initiated research while providing new research opportunities. To control the growth of continuing commitments and support planned new and expanded initiatives, the Fiscal Year 2001 request provides average cost increases of 2 percent over Fiscal Year 2000 for competing RPGs. Noncompeting RPGs will receive increases of 2 percent on average for recurring costs. This strategy will ensure that NIH can maintain a healthy number of new awards, especially for first time researchers.
Promises for advancement in medical research are dependent on a continuing supply of new investigators with new ideas. In the Fiscal Year 2001 request, NIA will support 526 pre- and postdoctoral trainees in full-time training positions. Stipends will increase by 2.2 percent over Fiscal Year 2000 levels.
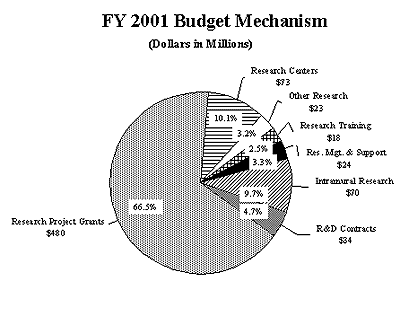
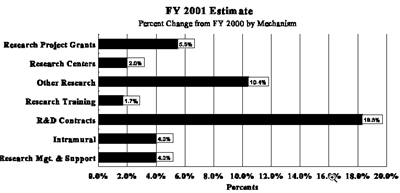
The Fiscal Year 2001 request includes funding for 64 research centers, 193 other research grants, including 164 clinical career awards, and 54 R&D contracts. The mechanism distribution by dollars and percent change are displayed below:
NATIONAL INSTITUTE ON AGING
Total by Mechanism
(Dollars in Thousands)
|
Mechanisms
Research Project Grants |
FY 1999 |
FY 2000 |
FY 2001 |
Percent
Change
From
FY 2000 |
|
Budget Number |
Authority
Amount |
Estimate |
Estimate |
|
Number |
Amount |
Number |
Amount |
|
Noncompeting |
685 |
$235,256 |
736 |
$296,933 |
836 |
$344,776 |
16.1% |
|
Admin Supplements |
(96) |
5,692 |
(110) |
7,210 |
(110) |
7,470 |
3.6% |
|
Competing |
398 |
130,919 |
389 |
138,093 |
315 |
113,972 |
-17.5% |
|
Subtotal |
1,083 |
371,867 |
1,125 |
442,236 |
1,151 |
466,218 |
5.4% |
|
SBIR/STTR |
52 |
12,988 |
60 |
15,006 |
65 |
16,243 |
8.2% |
|
Subtotal, RPG |
1,135 |
384,855 |
1,185 |
457,242 |
1,216 |
482,461 |
5.5% |
|
Research Centers |
64 |
70,520 |
64 |
71,825 |
64 |
73,263 |
2.0% |
|
Other Research |
186 |
19,356 |
194 |
20,651 |
194 |
22,815 |
10.5% |
|
Training |
526 |
16,319 |
526 |
17,300 |
526 |
17,589 |
1.7% |
|
R&D Contracts |
54 |
27,680 |
54 |
29,077 |
54 |
34,390 |
18.3% |
| (SBIR/STTR Contracts) |
(1) |
(497) |
(1) |
(497) |
0 |
0 |
-100.0% |
|
Intramural Research |
|
58,891 |
|
68,232 |
|
70,956 |
4.0% |
|
Rsch Mgmt & Support |
|
22,099 |
|
23,534 |
|
24,475 |
4.0% |
|
TOTAL |
|
599,720 |
|
687,861 |
|
725,949 |
5.5% |
Total amounts include funding for AIDS: FY99-$2,068; FY00-$4,143; FY01-$4,298
1. National Center for Health Statistics. Health, United States, 1999 With Health and Aging Chartbook. page 30, Hyattsville, MD: 1999.
2. National Center for Health Statistics. Health, United States, 1999 With Health and Aging Chartbook. page 22, Hyattsville, MD: 1999.
3. Small, GW, Rabine, PV, Barry, PP, et al. Diagnosis and Treatment of Alzheimer Disease and Related Disorders. JAMA 16: 1363-1371, 1997.
4. Ernst, RL, Hay, JW, Fenn, C, Tinklenberg, J and Yesavage, J. Cognitive Function and the Costs of Alzheimer's Disease, Arch Neurol 54:687-693, 1997.
5. Inouye SK, Bogardus ST, Charpentier PA, Leo-Sumers L, Acampora D, Holford TR, Cooney LM. A Multicomponent Intervention to Prevent Delirium in Hospitalized Older Patients. NEJM 340:9; 669-676. March 1999.
6. Fantl JA, Newman DK, Coiling J, et.al. Urinary Incontinence in Adults: Acute and Chronic Management, Rockville, MD: US Dept of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1996. Clinical Practice Guideline No. 2, 1996 Update, AHCPR publication No. 96-0682.
7. Wagner TH, Hu TW. Economic Costs of Urinary Incontinence in 1995. Urology. 51:3;355-361. 1998.
8. Day, JC, Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 - 2050, U.S. Bureau of the Census, Current Population Reports, P25-1130, U.S. Government Printing Office, Washington, DC 1996.
9. National Center for Health Statistics. Health, United States. 1999 With Health and Aging Chartbook. Table 33, pg. 156. Hyattsville, MD: 1999.
