More on How HIV Causes AIDS
A significant component of the research effort of the National Institute of Allergy and Infectious Diseases (NIAID) is devoted to the pathogenesis of HIV (human immunodeficiency virus) disease. Studies on pathogenesis address the complex mechanisms that result in the destruction of the immune system of an HIV-infected person. A detailed understanding of HIV and how it establishes infection and causes AIDS (acquired immunodeficiency syndrome) is crucial to identifying and developing effective drugs and vaccines to fight HIV and AIDS. This fact sheet summarizes the state of knowledge in this area.
(Scientific terms printed in bold-faced type are defined in the Glossary at the end of this document.)
Overview
Untreated HIV disease is characterized by a gradual deterioration of immune function. Most notably, crucial immune cells called CD4 positive (CD4+) T cells are disabled and killed during the typical course of infection. These cells, sometimes called "T-helper cells," play a central role in the immune response, signaling other cells in the immune system to perform their special functions.
A healthy, uninfected person usually has 800 to 1,200 CD4+ T cells per cubic millimeter (mm3) of blood. During untreated HIV infection, the number of these cells in a person's blood progressively declines. When the CD4+ T cell count falls below 200/mm3, a person becomes particularly vulnerable to the opportunistic infections and cancers that typify AIDS, the end stage of HIV disease. People with AIDS often suffer infections of the lungs, intestinal tract, brain, eyes, and other organs, as well as debilitating weight loss, diarrhea, neurologic conditions, and cancers such as Kaposi's sarcoma and certain types of lymphomas.
Most scientists think that HIV causes AIDS by directly inducing the death of CD4+ T cells or interfering with their normal function, and by triggering other events that weaken a person's immune function. For example, the network of signaling molecules that normally regulates a person's immune response is disrupted during HIV disease, impairing a person's ability to fight other infections. The HIV-mediated destruction of the lymph nodes and related immunologic organs also plays a major role in causing the immunosuppression seen in people with AIDS. Immunosuppression by HIV is confirmed by the fact that medicines, which interfere with the HIV lifecycle, preserve CD4+ T cells and immune function as well as delay clinical illness.
Scope of the HIV Epidemic
Although HIV was first identified in 1983, studies of previously stored blood samples indicate that the virus entered the U.S. population sometime in the late 1970s. In the United States, 886,575 cases of AIDS, and 501,669 deaths among people with AIDS had been reported to the Centers for Disease Control and Prevention (CDC) by the end of 2002. Approximately 40,000 new HIV infections occur each year in the United States, 70 percent of them among men and 30 percent among women. Of the new infections, approximately 40 percent are from male-to-male contact, 30 percent from heterosexual contact, and 25 percent from injection drug use. Minority groups in the United States have also been disproportionately affected by the epidemic.
Worldwide, an estimated 38 million people were living with HIV/AIDS as of December 2003, according to the Joint United Nations Programme on HIV/AIDS (UNAIDS) . Through 2003, cumulative AIDS-associated deaths worldwide numbered more than 20 million. Globally, approximately 5 million new HIV infections and approximately 3 million AIDS-related deaths, including an estimated 490,000 children under 15 years old, occurred in the year 2003 alone.
HIV is a retrovirus
HIV belongs to a class of viruses called retroviruses. Retroviruses are RNA (ribonucleic acid) viruses, and in order to replicate (duplicate). they must make a DNA (deoxyribonucleic acid) copy of their RNA. It is the DNA genes that allow the virus to replicate.
Like all viruses, HIV can replicate only inside cells, commandeering the cell's machinery to reproduce. Only HIV and other retroviruses, however, once inside a cell, use an enzyme called reverse transcriptase to convert their RNA into DNA, which can be incorporated into the host cell's genes.
Slow viruses
HIV belongs to a subgroup of retroviruses known as lentiviruses, or "slow" viruses. The course of infection with these viruses is characterized by a long interval between initial infection and the onset of serious symptoms.
Other lentiviruses infect nonhuman species. For example, the feline immunodeficiency virus (FIV) infects cats and the simian immunodeficiency virus (SIV) infects monkeys and other nonhuman primates. Like HIV in humans, these animal viruses primarily infect immune system cells, often causing immune deficiency and AIDS-like symptoms. These viruses and their hosts have provided researchers with useful, albeit imperfect, models of the HIV disease process in people.
Structure of HIV
The viral envelope
HIV has a diameter of 1/10,000 of a millimeter and is spherical in shape. The outer coat of the virus, known as the viral envelope, is composed of two layers of fatty molecules called lipids, taken from the membrane of a human cell when a newly formed virus particle buds from the cell. Evidence from NIAID-supported research indicates that HIV may enter and exit cells through special areas of the cell membrane known as "lipid rafts." These rafts are high in cholesterol and glycolipids and may provide a new target for blocking HIV.
Embedded in the viral envelope are proteins from the host cell, as well as 72 copies (on average) of a complex HIV protein (frequently called "spikes") that protrudes through the surface of the virus particle (virion). This protein, known as Env, consists of a cap made of three molecules called glycoprotein (gp) 120, and a stem consisting of three gp41 molecules that anchor the structure in the viral envelope. Much of the research to develop a vaccine against HIV has focused on these envelope proteins.
The viral core
Within the envelope of a mature HIV particle is a bullet-shaped core or capsid, made of 2,000 copies of another viral protein, p24. The capsid surrounds two single strands of HIV RNA, each of which has a copy of the virus's nine genes. Three of these genes, gag, pol, and env, contain information needed to make structural proteins for new virus particles. The env gene, for example, codes for a protein called gp160 that is broken down by a viral enzyme to form gp120 and gp41, the components of Env.
Six regulatory genes, tat, rev, nef, vif, vpr, and vpu, contain information necessary to produce proteins that control the ability of HIV to infect a cell, produce new copies of virus, or cause disease. The protein encoded by nef, for instance, appears necessary for the virus to replicate efficiently, and the vpu-encoded protein influences the release of new virus particles from infected cells. Recently, researchers discovered that Vif (the protein encoded by the vif gene) interacts with an antiviral defense protein in host cells (APOBEC3G), causing inactivation of the antiviral effect and enhancing HIV replication. This interaction may serve as a new target for antiviral drugs.
The ends of each strand of HIV RNA contain an RNA sequence called the long terminal repeat (LTR). Regions in the LTR act as switches to control production of new viruses and can be triggered by proteins from either HIV or the host cell.
The core of HIV also includes a protein called p7, the HIV nucleocapsid protein. Three enzymes carry out later steps in the virus's life cycle: reverse transcriptase, integrase, and protease. Another HIV protein called p17, or the HIV matrix protein, lies between the viral core and the viral envelope.
Replication Cycle of HIV
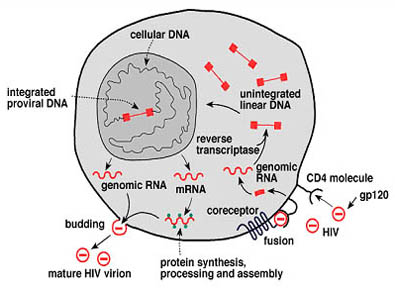
Entry of HIV into cells
Infection typically begins when an HIV particle, which contains two copies of the HIV RNA, encounters a cell with a surface molecule called cluster designation 4 (CD4). Cells carrying this molecule are known as CD4+ cells.
One or more of the virus's gp120 molecules binds tightly to CD4 molecule(s) on the cell's surface. The binding of gp120 to CD4 results in a conformational change in the gp120 molecule allowing it to bind to a second molecule on the cell surface known as a co-receptor. The envelope of the virus and the cell membrane then fuse, leading to entry of the virus into the cell. The gp41 of the envelope is critical to the fusion process. Drugs that block either the binding or the fusion process are being developed and tested in clinical trials. The Food and Drug Administration (FDA) has approved one of the so-called fusion inhibitors, T20, for use in HIV-infected people.
Studies have identified multiple coreceptors for different types of HIV strains. These coreceptors are promising targets for new anti-HIV drugs, some of which are now being tested in preclinical and clinical studies. Agents that block the co-receptors are showing particular promise as potential microbicides that could be used in gels or creams to prevent HIV transmission. In the early stage of HIV disease, most people harbor viruses that use, in addition to CD4, a receptor called CCR5 to enter their target cells. With disease progression, the spectrum of co-receptor usage expands in approximately 50 percent of patients to include other receptors, notably a molecule called CXCR4. Virus that uses CCR5 is called R5 HIV and virus that uses CXCR4 is called X4 HIV.
Although CD4+ T cells appear to be the main targets of HIV, other immune system cells with and without CD4 molecules on their surfaces are infected as well. Among these are long-lived cells called monocytes and macrophages , which apparently can harbor large quantities of the virus without being killed, thus acting as reservoirs of HIV. CD4+ T cells also serve as important reservoirs of HIV; a small proportion of these cells harbor HIV in a stable, inactive form. Normal immune processes may activate these cells, resulting in the production of new HIV virions.
Cell-to-cell spread of HIV also can occur through the CD4-mediated fusion of an infected cell with an uninfected cell.
 |
| Credit: NIAID. |
Reverse transcription
In the cytoplasm of the cell, HIV reverse transcriptase converts viral RNA into DNA, the nucleic acid form in which the cell carries its genes. Fifteen of the 26 antiviral drugs approved in the United States for treating people with HIV infection work by interfering with this stage of the viral life cycle.
Integration
The newly made HIV DNA moves to the cell's nucleus, where it is spliced into the host's DNA with the help of HIV integrase. HIV DNA that enters the DNA of the cell is called a provirus. Several drugs that target the integrase enzyme are in the early stages of development and are being investigated for their potential as antiretroviral agents.
Transcription
For a provirus to produce new viruses, RNA copies must be made that can be read by the host cell's protein-making machinery. These copies are called messenger RNA (mRNA), and production of mRNA is called transcription, a process that involves the host cell's own enzymes. Viral genes in concert with the cellular machinery control this process; the tat gene, for example, encodes a protein that accelerates transcription. Genomic RNA is also transcribed for later incorporation in the budding virion (see below).
Cytokines, proteins involved in the normal regulation of the immune response, also may regulate transcription. Molecules such as tumor necrosis factor (TNF)-alpha and interleukin (IL)-6, secreted in elevated levels by the cells of HIV-infected people, may help to activate HIV proviruses. Other infections, by organisms such as Mycobacterium tuberculosis, also may enhance transcription by inducing the secretion of cytokines.
Translation
After HIV mRNA is processed in the cell's nucleus, it is transported to the cytoplasm. HIV proteins are critical to this process; for example, a protein encoded by the rev gene allows mRNA encoding HIV structural proteins to be transferred from the nucleus to the cytoplasm. Without the rev protein, structural proteins are not made. In the cytoplasm, the virus co-opts the cell's protein-making machinery-including structures called ribosomes-to make long chains of viral proteins and enzymes, using HIV mRNA as a template. This process is called translation.
Assembly and budding
Newly made HIV core proteins, enzymes, and genomic RNA gather inside the cell and an immature viral particle forms and buds off from the cell, acquiring an envelope that includes both cellular and HIV proteins from the cell membrane. During this part of the viral life cycle, the core of the virus is immature and the virus is not yet infectious. The long chains of proteins and enzymes that make up the immature viral core are now cut into smaller pieces by a viral enzyme called protease.
This step results in infectious viral particles. Drugs called protease inhibitors interfere with this step of the viral life cycle. FDA has approved eight such drugs-saquinavir, ritonavir, indinavir, amprenavir, nelfinavir, fosamprenavir, atazanavir, and lopinavir-for marketing in the United States. Recently, an HIV inhibitor that targets a unique step in the viral life cycle, very late in the process of viral maturation, has been identified and is currently undergoing further development.
Recently, researchers have discovered that virus budding from the host cell is much more complex than previously thought. Binding between the HIV Gag protein and molecules in the cell directs the accumulation of HIV components in special intracellular sacks, called multivesicular bodies (MVB), that normally function to carry proteins out of the cell. In this way, HIV actively hitch-hikes out of the cell in the MVB by hijacking normal cell machinery and mechanisms. Discovery of this budding pathway has revealed several potential points for intervening in the viral replication cycle.
Transmission of HIV
Among adults, HIV is spread most commonly during sexual intercourse with an infected partner. During intercourse, the virus can enter the body through the mucosal linings of the vagina, vulva, penis, or rectum or, rarely, via the mouth and possibly the upper gastrointestinal tract after oral sex. The likelihood of transmission is increased by factors that may damage these linings, especially other sexually transmitted infections that cause ulcers or inflammation.
Research suggests that immune system cells of the dendritic cell type, which live in the mucosa, may begin the infection process after sexual exposure by binding to and carrying the virus from the site of infection to the lymph nodes where other immune system cells become infected. A molecule on the surface of dendritic cells, DC-SIGN, may be critical for this transmission process.
HIV also can be transmitted by contact with infected blood, most often by the sharing of needles or syringes contaminated with minute quantities of blood containing the virus. The risk of acquiring HIV from blood transfusions is extremely small in the United States, as all blood products in this country are screened routinely for evidence of the virus.
Almost all HIV-infected children in the United States get the virus from their mothers before or during birth. In the United States, approximately 25 percent of pregnant HIV-infected women not receiving antiretroviral therapy have passed on the virus to their babies. In 1994, researchers showed that a specific regimen of the drug AZT (zidovudine) can reduce the risk of transmission of HIV from mother to baby by two-thirds. The use of combinations of antiretroviral drugs and simpler drug regimens has further reduced the rate of mother-to-child HIV transmission in the United States.
In developing countries, cheap and simple antiviral drug regimens have been proven to significantly reduce mother-to-child transmission at birth in resource-poor settings. Unfortunately, the virus also may be transmitted from an HIV-infected mother to her infant via breastfeeding. Moreover, due to the use of medicines to prevent transmission at delivery, breastfeeding may become the most common mode of HIV infection in infants. Thus, development of affordable alternatives to breastfeeding is greatly needed.
Early Events in HIV Infection
Once it enters the body, HIV infects a large number of CD4+ cells and replicates rapidly. During this acute or primary phase of infection, the blood contains many viral particles that spread throughout the body, seeding various organs, particularly the lymphoid organs.
Two to 4 weeks after exposure to the virus, up to 70 percent of HIV-infected people suffer flu-like symptoms related to the acute infection. Their immune system fights back with killer T cells (CD8+ T cells) and B-cell-produced antibodies , which dramatically reduce HIV levels. A person's CD4+ T cell count may rebound somewhat and even approach its original level. A person may then remain free of HIV-related symptoms for years despite continuous replication of HIV in the lymphoid organs that had been seeded during the acute phase of infection.
One reason that HIV is unique is the fact that despite the body's aggressive immune responses, which are sufficient to clear most viral infections, some HIV invariably escapes. This is due in large part to the high rate of mutations that occur during the process of HIV replication. Even when the virus does not avoid the immune system by mutating, the body's best soldiers in the fight against HIV-certain subsets of killer T cells that recognize HIV-may be depleted or become dysfunctional.
In addition, early in the course of HIV infection, people may lose HIV-specific CD4+ T cell responses that normally slow the replication of viruses. Such responses include the secretion of interferons and other antiviral factors, and the orchestration of CD8+ T cells.
Finally, the virus may hide within the chromosomes of an infected cell and be shielded from surveillance by the immune system. Such cells can be considered as a latent reservoir of the virus. Because the antiviral agents currently in our therapeutic arsenal attack actively replicating virus, they are not effective against hidden, inactive viral DNA (so-called provirus). New strategies to purge this latent reservoir of HIV have become one of the major goals for current research efforts.
Course of HIV Infection
Among people enrolled in large epidemiologic studies in Western countries, the median time from infection with HIV to the development of AIDS-related symptoms has been approximately 10 to 12 years in the absence of antiretroviral therapy. Researchers, however, have observed a wide variation in disease progression. Approximately 10 percent of HIV-infected people in these studies have progressed to AIDS within the first 2 to 3 years following infection, while up to 5 percent of individuals in the studies have stable CD4+ T cell counts and no symptoms even after 12 or more years.
Factors such as age or genetic differences among individuals, the level of virulence of an individual strain of virus, and co-infection with other microbes may influence the rate and severity of disease progression. Drugs that fight the infections associated with AIDS have improved and prolonged the lives of HIV-infected people by preventing or treating conditions such as Pneumocystis carinii pneumonia, cytomegalovirus disease, and diseases caused by a number of fungi.
HIV co-receptors and disease progression
Recent research has shown that most infecting strains of HIV use a co-receptor molecule called CCR5, in addition to the CD4 molecule, to enter certain of its target cells. HIV-infected people with a specific mutation in one of their two copies of the gene for this receptor may have a slower disease course than people with two normal copies of the gene. Rare individuals with two mutant copies of the CCR5 gene appear, in most cases, to be completely protected from HIV infection. Mutations in the gene for other HIV co-receptors also may influence the rate of disease progression.
Viral burden and disease progression
Numerous studies show that people with high levels of HIV in their bloodstream are more likely to develop new AIDS-related symptoms or die than those with lower levels of virus. For instance, in the Multicenter AIDS Cohort Study (MACS), investigators showed that the level of HIV in an untreated person's plasma 6 months to a year after infection-the so-called viral "set point"-is highly predictive of the rate of disease progression; that is, patients with high levels of virus are much more likely to get sicker faster than those with low levels of virus. The MACS and other studies have provided the rationale for providing aggressive antiretroviral therapy to HIV-infected people, as well as for routinely using newly available blood tests to measure viral load when initiating, monitoring, and modifying anti-HIV therapy.
Potent combinations of three or more anti-HIV drugs known as highly active antiretroviral therapy, or HAART, can reduce a person's "viral burden" (amount of virus in the circulating blood) to very low levels and in many cases delay the progression of HIV disease for prolonged periods. Before the introduction of HAART therapy, 85 percent of patients survived an average of 3 years following AIDS diagnosis. Today, 95 percent of patients who start therapy before they get AIDS survive on average 3 years following their first AIDS diagnosis. For those who start HAART after their first AIDS event, survival is still very high at 85 percent, averaging 3 years after AIDS diagnosis.
Antiretroviral regimens, however, have yet to completely and permanently suppress the virus in HIV-infected people. Recent studies have shown that, in addition to the latent HIV reservoir discussed above, HIV persists in a replication-competent form in resting CD4+ T cells even in people receiving aggressive antiretroviral therapy who have no readily detectable HIV in their blood. Investigators around the world are working to develop the next generation of anti-HIV drugs that can stop HIV, even in these biological scenarios.
A treatment goal, along with reduction of viral burden, is the reconstitution of the person's immune system, which may have become sufficiently damaged that it cannot replenish itself. Various strategies for assisting the immune system in this regard are being tested in clinical trials in tandem with HAART, such as the Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT) trial exploring the effects of the T cell growth factor, IL-2.
HIV is Active in the Lymph Nodes
Although HIV-infected people often show an extended period of clinical latency with little evidence of disease, the virus is never truly completely latent although individual cells may be latently infected. Researchers have shown that even early in disease, HIV actively replicates within the lymph nodes and related organs, where large amounts of virus become trapped in networks of specialized cells with long, tentacle-like extensions. These cells are called follicular dendritic cells (FDCs). FDCs are located in hot spots of immune activity in lymphoid tissue called germinal centers. They act like flypaper, trapping invading pathogens (including HIV) and holding them until B cells come along to start an immune response.
Over a period of years, even when little virus is readily detectable in the blood, significant amounts of virus accumulate in the lymphoid tissue, both within infected cells and bound to FDCs. In and around the germinal centers, numerous CD4+ T cells are probably activated by the increased production of cytokines such as TNF-alpha and IL-6 by immune system cells within the lymphoid tissue. Activation allows uninfected cells to be more easily infected and increases replication of HIV in already infected cells.
While greater quantities of certain cytokines such as TNF-alpha and IL-6 are secreted during HIV infection, other cytokines with key roles in the regulation of normal immune function may be secreted in decreased amounts. For example, CD4+ T cells may lose their capacity to produce IL-2, a cytokine that enhances the growth of other T cells and helps to stimulate other cells' response to invaders. Infected cells also have low levels of receptors for IL-2, which may reduce their ability to respond to signals from other cells.
Breakdown of lymph node architecture
Ultimately, with chronic cell activation and secretion of inflammatory cytokines, the fine and complex inner structure of the lymph node breaks down and is replaced by scar tissue. Without this structure, cells in the lymph node cannot communicate and the immune system cannot function properly. Investigators also have reported recently that this scarring reduces the ability of the immune system to replenish itself following antiretroviral therapy that reduces the viral burden.
Role of CD8+ T Cells
CD8+ T cells are critically important in the immune response to HIV. These cells attack and kill infected cells that are producing virus. Thus, vaccine efforts are directed toward eliciting or enhancing these killer T cells, as well as eliciting antibodies that will neutralize the infectivity of HIV.
CD8+ T cells also appear to secrete soluble factors that suppress HIV replication. Several molecules, including RANTES, MIP-1alpha, MIP-1beta, and MDC appear to block HIV replication by occupying the coreceptors necessary for many strains of HIV to enter their target cells. There may be other immune system molecules-including the so-called CD8 antiviral factor (CAF), the defensins (type of antimicrobials), and others yet undiscovered-that can suppress HIV replication to some degree.
Rapid Replication and Mutation of HIV
HIV replicates rapidly; several billion new virus particles may be produced every day. In addition, the HIV reverse transcriptase enzyme makes many mistakes while making DNA copies from HIV RNA. As a consequence, many variants or strains of HIV develop in a person, some of which may escape destruction by antibodies or killer T cells. Additionally, different strains of HIV can recombine to produce a wide range of variants.
During the course of HIV disease, viral strains emerge in an infected person that differ widely in their ability to infect and kill different cell types, as well as in their rate of replication. Scientists are investigating why strains of HIV from people with advanced disease appear to be more virulent and infect more cell types than strains obtained earlier from the same person. Part of the explanation may be the expanded ability of the virus to use other co-receptors, such as CXCR4.
Theories of Immune System Cell Loss in HIV Infection
Researchers around the world are studying how HIV destroys or disables CD4+ T cells, and many think that a number of mechanisms may occur simultaneously in an HIV-infected person. Data suggest that billions of CD4+ T cells may be destroyed every day, eventually overwhelming the immune system's capacity to regenerate.
Direct cell killing
Infected CD4+ T cells may be killed directly when large amounts of virus are produced and bud out from the cell surface, disrupting the cell membrane, or when viral proteins and nucleic acids collect inside the cell, interfering with cellular machinery.
Apoptosis
Infected CD4+ T cells may be killed when the regulation of cell function is distorted by HIV proteins, probably leading to cell suicide by a process known as programmed cell death or apoptosis. Recent reports indicate that apoptosis occurs to a greater extent in HIV-infected people, both in their bloodstream and lymph nodes. Apoptosis is closely associated with the aberrant cellular activation seen in HIV disease.
Uninfected cells also may undergo apoptosis. Investigators have shown in cell cultures that the HIV envelope alone or bound to antibodies sends an inappropriate signal to CD4+ T cells causing them to undergo apoptosis, even if not infected by HIV.
Innocent bystanders
Uninfected cells may die in an innocent bystander scenario: HIV particles may bind to the cell surface, giving them the appearance of an infected cell and marking them for destruction by killer T cells after antibody attaches to the viral particle on the cell. This process is called antibody-dependent cellular cytotoxicity.
Killer T cells also may mistakenly destroy uninfected cells that have consumed HIV particles and that display HIV fragments on their surfaces. Alternatively, because HIV envelope proteins bear some resemblance to certain molecules that may appear on CD4+ T cells, the body's immune responses may mistakenly damage such cells as well.
Anergy
Researchers have shown in cell cultures that CD4+ T cells can be turned off by activation signals from HIV that leaves them unable to respond to further immune stimulation. This inactivated state is known as anergy.
Damage to precursor cells
Studies suggest that HIV also destroys precursor cells that mature to have special immune functions, as well as the microenvironment of the bone marrow and the thymus needed for developing such cells. These organs probably lose the ability to regenerate, further compounding the suppression of the immune system.
Central Nervous System Damage
Although monocytes and macrophages can be infected by HIV, they appear to be relatively resistant to being killed by the virus. These cells, however, travel throughout the body and carry HIV to various organs, including the brain, which may serve as a hiding place or "reservoir" for the virus that may be relatively resistant to most anti-HIV drugs.
Neurologic manifestations of HIV disease are seen in up to 50 percent of HIV-infected people, to varying degrees of severity. People infected with HIV often experience
- Cognitive symptoms, including impaired short-term memory, reduced concentration, and mental slowing
- Motor symptoms such as fine motor clumsiness or slowness, tremor, and leg weakness
- Behavioral symptoms including apathy, social withdrawal, irritability, depression, and personality change
More serious neurologic manifestations in HIV disease typically occur in patients with high viral loads, generally when a person has advanced HIV disease or AIDS.
Neurologic manifestations of HIV disease are the subject of many research projects. Current evidence suggests that although nerve cells do not become infected with HIV, supportive cells within the brain, such as astrocytes and microglia (as well as monocyte/macrophages that have migrated to the brain) can be infected with the virus. Researchers postulate that infection of these cells can cause a disruption of normal neurologic functions by altering cytokine levels, by delivering aberrant signals, and by causing the release of toxic products in the brain. The use of anti-HIV drugs frequently reduces the severity of neurologic symptoms, but in many cases does not, for reasons that are unclear. The impact of long-term therapy and long-term HIV disease on neurologic function is also unknown and under intensive study.
Role of Immune Activation in HIV Disease
During a normal immune response, many parts of the immune system are mobilized to fight an invader. CD4+ T cells, for instance, may quickly multiply and increase their cytokine secretion, thereby signaling other cells to perform their special functions. Scavenger cells called macrophages may double in size and develop numerous organelles , including lysosomes that contain digestive enzymes used to process ingested pathogens. Once the immune system clears the foreign antigen, it returns to a relative state of quiescence.
Paradoxically, although it ultimately causes immune deficiency, HIV disease for most of its course is characterized by immune system hyperactivation, which has negative consequences. As noted above, HIV replication and spread are much more efficient in activated CD4+ cells. Chronic immune system activation during HIV disease also may result in a massive stimulation of B cells, impairing the ability of these cells to make antibodies against other pathogens.
Chronic immune activation also can result in apoptosis, and an increased production of cytokines that not only may increase HIV replication but also have other deleterious effects. Increased levels of TNF-alpha, for example, may be at least partly responsible for the severe weight loss or wasting syndrome seen in many HIV-infected people.
The persistence of HIV and HIV replication plays an important role in the chronic state of immune activation seen in HIV-infected people. In addition, researchers have shown that infections with other organisms activate immune system cells and increase production of the virus in HIV-infected people. Chronic immune activation due to persistent infections, or the cumulative effects of multiple episodes of immune activation and bursts of virus production, likely contribute to the progression of HIV disease.
New Clinical Signs of HIV in the Era of HAART Therapy
The clinical spectrum of disease among people with HIV has changed dramatically in the era of HAART. NIAID and its grantees are actively studying the new clinical syndrome of disease among persons on long term-therapy. Research is concentrating on the impact of HIV over the long term, the toxicity of the medicines used to control HIV, and the effects of aging on HIV disease progression. People with HIV have a variety of conditions including diabetes, heart disease, neurocognitive decline, and cancers that may, or may not, be directly due to HIV or its treatment. Long-term studies of people with HIV in the United States and abroad are underway.
NIAID Research on the Pathogenesis of AIDS
NIAID-supported scientists conduct research on HIV pathogenesis in laboratories on the campus of the National Institutes of Health (NIH) in Bethesda, Maryland; at the Institute's Rocky Mountain Laboratories in Hamilton, Montana; and at universities and medical centers in the United States and abroad.
An NIAID-supported resource, the NIH AIDS Research and Reference Reagent Program , in collaboration with the World Health Organization, provides critically needed AIDS-related research materials free to qualified researchers around the world.
The NIH Centers for AIDS Research , supported by NIAID in collaboration with six other NIH Institutes, fosters and facilitates development of infrastructure and interdisciplinary collaboration of HIV researchers at major medical and research centers across the United States.
In addition, the Institute convenes groups of investigators and advisory committees to exchange scientific information, clarify research priorities, and bring research needs and opportunities to the attention of the scientific community.
Glossary
antibodies - infection-fighting protein molecules in blood or secretory fluids that tag, neutralize, and help destroy pathogenic microorganisms such as viruses.
apoptosis - cellular suicide, also known as programmed cell death. HIV may induce apoptosis in both infected and uninfected immune system cells.
B cells - white blood cells of the immune system that produce infection-fighting proteins called antibodies.
CD4+ T cells - white blood cells that orchestrate the immune response, signaling other cells in the immune system to perform their special functions. Also known as T helper cells, these cells are killed or disabled during HIV infection.
CD8+ T cells - white blood cells that kill cells infected with HIV or other viruses, or transformed by cancer. These cells also secrete soluble molecules that may suppress HIV without killing infected cells directly.
cytokines - proteins used for communication by cells of the immune system. Central to the normal regulation of the immune response.
cytoplasm - the living matter within a cell.
dendritic cells - immune system cells with long, tentacle-like branches. Some of these are specialized cells at the mucosa that may bind to HIV following sexual exposure and carry the virus from the site of infection to the lymph nodes. See also follicular dendritic cells.
enzyme - a protein that accelerates a specific chemical reaction without altering itself.
follicular dendritic cells (FDCs) - cells found in the germinal centers (B cell areas) of lymphoid organs. FDCs have thread-like tentacles that form a web-like network to trap invaders and present them to B cells, which then make antibodies to attack the invaders.
germinal centers - structures within lymphoid tissues that contain FDCs and B cells, and in which immune responses are initiated.
gp41 - glycoprotein 41, a protein embedded in the outer envelope of HIV. Plays a key role in HIV's infection of CD4+ T cells by facilitating the fusion of the viral and cell membranes.
gp120 - glycoprotein 120, a protein that protrudes from the surface of HIV and binds to CD4+ T cells.
gp160 - glycoprotein 160, an HIV precursor protein that is cleaved by the HIV protease enzyme into gp41 and gp120.
immune deficiency - the inability of the immune system to work properly, resulting in susceptibility to disease.
immunosuppression - immune system response to foreign invaders such as HIV is reduced
integrase - an HIV enzyme used by the virus to integrate its genetic material into the host cell's DNA.
Kaposi's sarcoma - a type of cancer characterized by abnormal growths of blood vessels that develop into purplish or brown lesions.
killer T cells - see CD8+ T cells.
lentivirus - "slow" virus characterized by a long interval between infection and the onset of symptoms. HIV is a lentivirus as is the simian immunodeficiency virus (SIV), which infects nonhuman primates.
LTR - long terminal repeat, the RNA sequences repeated at both ends of HIV's genetic material. These regulatory switches may help control viral transcription.
lymphoid organs - include tonsils, adenoids, lymph nodes, spleen, and other tissues. Act as the body's filtering system, trapping invaders and presenting them to squadrons of immune cells that congregate there.
macrophage - a large immune system cell that devours invading pathogens and other intruders. Stimulates other immune system cells by presenting them with small pieces of the invaders.
microbes - microscopic living organisms, including viruses, bacteria, fungi, and protozoa.
monocyte - a circulating white blood cell that develops into a macrophage when it enters tissues.
opportunistic infection - an illness caused by an organism that usually does not cause disease in a person with a normal immune system. People with advanced HIV infection suffer opportunistic infections of the lungs, brain, eyes, and other organs.
organelles - small structures inside a cell, generally bounded by membranes.
pathogenesis - the production or development of a disease. May be influenced by many factors, including the infecting microbe and the host's immune response.
pathogens - disease-causing organisms.
protease - an HIV enzyme used to cut large HIV proteins into smaller ones needed for the assembly of an infectious virus particle.
provirus - DNA of a virus, such as HIV, that has been integrated into the genes of a host cell.
replicate - process by which a virus makes copies of itself.
retrovirus - HIV and other viruses that carry their genetic material in the form of RNA and that have the enzyme reverse transcriptase.
reverse transcriptase - the enzyme produced by HIV and other retroviruses that allows them to synthesize DNA from their RNA.
back to top