Appendix C: Human Embryonic Stem Cells and Human Embryonic Germ Cells
Methods for Growing Human Embryonic Stem Cells In Vitro
To grow cultures of human ES cells, Thomson and his collaborators used 36 fresh or frozen embryos generated in IVF laboratories from couples undergoing treatment for infertility. From the 14 embryos that developed to the blastocyst stage, they established 5 human ES cell lines—H1, H7, H9, H13 and H14 [35]. Four of the 5 lines were derived from frozen embryos provided to Thomson's laboratory by Josef Itskovitz-Eldor, of the Rambam Medical Center in Haifa, Israel. The ES cell line from the fifth, fresh embryo was derived from an embryo donated in Wisconsin.
To generate human ES cell cultures, cells from the inner cell mass of a human blastocyst were cultured in a multi-step process. The pluripotent cells of the inner cell mass were separated from the surrounding trophectoderm by immunosurgery, the antibody-mediated dissolution of the trophectoderm. The inner cell masses were plated in culture dishes containing growth medium supplemented with fetal bovine serum on feeder layers of mouse embryonic fibroblasts that had been gamma-irradiated to prevent their replication. After 9 to 15 days, when inner cell masses had divided and formed clumps of cells, cells from the periphery of the clumps were chemically or mechanically dissociated and replated in the same culture conditions. Colonies of apparently homogeneous cells were selectively removed, mechanically dissociated, and replated. These were expanded and passaged, thus creating a cell line. None of the initial 5 human ES cell lines generated in this manner was derived clonally (cloned from a single cell and are, therefore, genetically identical) [35] (see Figure C.1. Techniques for Generating Embryonic Stem Cell Cultures).
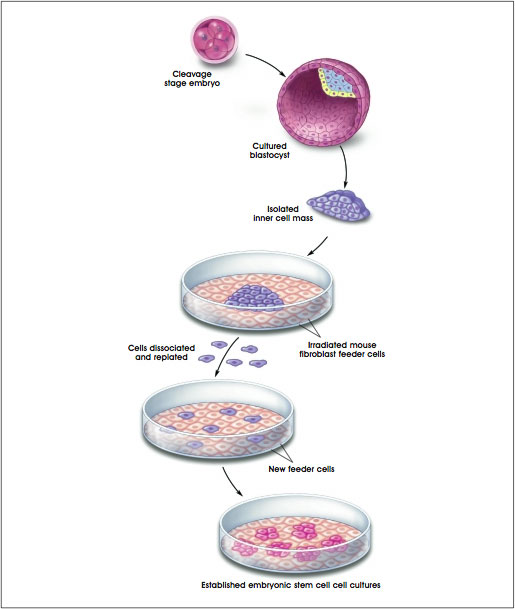
Figure C.1. Techniques for Generating Embryonic Stem Cell Cultures.
(© 2001 Terese Winslow, Caitlin Duckwall)
The five original human ES cell lines continued to divide without differentiating for 5 to 6 months [35]. Since then, the H9 line has divided for nearly two years in vitro, for more than 300 population doublings and has yielded two subclones, H9.1 and H9.2 [1]1. All the ES cell lines express high levels of telomerase [1, 36], the enzyme that helps maintain telomeres which protect the ends of chromosomes. Telomerase activity and long telomeres are characteristic of proliferating cells in embryonic tissues and of germ cells. Human somatic cells, however, do not show telomerase activity and their telomeres are considerably shorter. Unlike ES cells, differentiated somatic cells also stop dividing in culture—a phenomenon called replicative senescence (see Figure C.2. Telomeres and Telomerase).
1 The H9.1 and H9.2 clonal cell lines were produced by first plating 105 of the parent H9 cells per well in tissue-culture plates. The culture medium contained KnockOut Dulbecco's modified minimal essential medium (a serum-free substitute for the 20% fetal bovine serum used in the 1998 experiments), and basic FGF, which is necessary to maintain cell proliferation and prevent differentiation. To generate clonal cell lines from individual H9 ES cells, 384 single cells were removed from these cultures and transferred individually to the wells of larger plates that contained non-dividing mouse embryonic fibroblasts (MEF) as feeder layers. The single ES cells proliferated and, every 7 days, were dissociated and replated, a process that generate two clonal cell lines, H9.1 and H9.2.
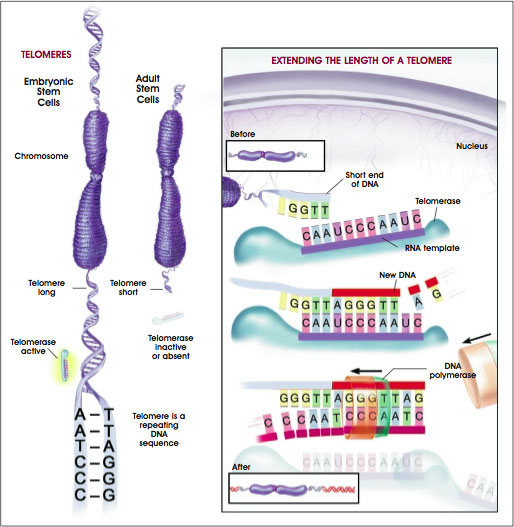
Figure C.2. Telomeres and Telomerase. A telomere is a repeating sequence of double-stranded DNA located at the ends of chromosomes. Greater telomere length is associated with immortalized cell lines such as embryonic stem cells and cancer cells. As cells divide and differentiate throughout the lifespan of an organism or cell line, the telomeres become progressively shortened and lose the ability to maintain their length. Telomerase is an enzyme that lengthens telomeres by adding on repeating sequences of DNA. Telomerase binds to the ends of the telomere via an RNA template that is used for the attachment of a new strand of DNA. Telomerase adds several repeated DNA sequences then releases and a second enzyme, DNA Polymerase, attaches the opposite or complementary strand of DNA completing the double stranded extension of the chromosome ends. High levels of telomerase activity are detected in embryonic stem cells and cancer cells, whereas little or no telomerase activity is present in most mature, differentiated cell types. The functions of telomeres and telomerase appear to be important in cell division, normal development, and aging.
(© 2001 Terese Winslow)
Three of the human ES cell lines generated by Thomson were XY (male) and two were XX (female); all maintained a normal karyotype. Like monkey ES cells [34], human ES cells express a panel of surface makers that include the stage-specific embryonic antigens SSEA-3 and SSEA-4, as well as TRA-1–60, TRA-1–81, and alkaline phosphatase [14, 25, 26, 35]. Mouse ES cells do not express SSEA-3 or SSEA-4; they express SSEA-1, which human and monkey ES cells do not. Human ES cells also express the transcription factor Oct-4 [26], as mouse ES cells do.
A somewhat different technique for deriving and culturing human ES cells has now been reported by investigators in Singapore and Australia. The human blastocysts are cultured in Singapore, where monolayer cultures of human ES cells growing on feeder layers are prepared. The primary cultures are shipped to Australia, where the colonies of growing cells are dissociated mechanically and replated. LIF and fetal bovine serum are added to the growth medium. The cells do not grow well without serum, although it is not clear that LIF has any effect. Under these in vitro conditions, the ES cells tend to clump and differentiate spontaneously as they are passaged. In vivo, after injection into the testes of immunocompromised mice, the ES cells differentiate into bone, cartilage, squamous and cuboidal epithelium, neural cells, glandular epithelium, and striated muscle [25, 26]. Six human ES cell lines have been generated from 12 blastocysts, a high yield by any standard. The original two cell lines were generated from fresh embryos; the other four cells lines were generated from frozen embryos [23].
Recent reports have identified additional human ES cell lines that have been developed. New derivations have been conducted from the blastocyst of frozen embryos at two centers in India (National Centre for Biological Sciences, University of Agriculture Sciences, Bangalore; Harkishondas Hospital in collaboration with Reliance Biotechnology, Bombay). They used derivation techniques that differ from those of the Thomson laboratory including the use of laser ablation for the removal of the inner cell mass [22, 24, 33, 36]. An additional preparation of human ES cell lines has been conducted in San Francisco [10]. There are no publications to date on these cell lines and the extent of the research being conducted is not known.
By several criteria, all of the human ES cell lines generated to date are pluripotent. When injected under the skin or into the testes of immunocompromised mice—an in vivo method of determining pluripotency—the human ES cells form teratomas that contain derivatives of all three primary germ layers. When allowed to differentiate in vitro (by culturing the cells in the absence of MEF feeder layers), the human ES cells differentiate spontaneously. Subsequent studies indicate that in vitro differentiation of these human ES cell lines is extensive; the cells can generate many cell types that are derived from all three primary germ layers [1, 14, 25, 26]. However, the extent to which these human ES cell lines will differentiate in vitro does not match their more extensive differentiation capability in vivo (in teratomas) [19].
Methods for the Derivation and Culture of Human Embryonic Germ Cells
To derive cultures of human embryonic germ (EG)-like cells, Gearhart and his colleagues grew cells from 38 initial cultures of primordial germ cells (PGC), which were obtained from the gonadal ridge and mesentery of 5 to 9-week gestation fetal tissue. (PGCs give rise to the germ cells, eggs and sperm, in the adult.) The PGCs were mechanically and chemically dissociated, then plated on a feeder layer of non-dividing, mouse STO fibroblasts in growth medium supplemented with fetal bovine serum [31]. Unlike the growth conditions initially reported for human ES cells [35], the medium for human PGCs cells also contained the cytokine, leukemia inhibitory factor (LIF), a mitogen (basic fibroblast growth factor, bFGF), and forskolin.
After one to three weeks in vitro, the human PGCs had formed dense, multilayered colonies of cells that resembled mouse ES or EG cells. Cells in these colonies expressed SSEA-1, SSEA-3, SSEA-4, TRA1–60, TRA-1–81, and alkaline phosphatase. A small, variable percentage (1 to 20 %) of the PGC-derived cell colonies spontaneously formed embryoid bodies. The growth medium for embryoid body cultures lacked LIF, bFGF, and forskolin. The embryoid bodies were collected from the cultures and either examined for the cell types they contained, or replated into single wells of a tissue culture plate for 14 days. The range of cell types in the human PGC-derived embryoid bodies included derivatives of all three embryonic germ layers—endoderm, mesoderm, and ectoderm—based on the appearance of the cells and the surface markers they expressed. This result was interpreted to mean that the PGC-derived cells were pluripotent, however, it was not possible to demonstrate pluripotency in vivo by generating the formation of teratomas in mice [31].
In their next series of experiments, Gearhart and his collaborators devised methods for growing stem cells derived from human EG cells. The process requires the generation of embryoid bodies, which form spontaneously from EG cells that remain attached to the substrate. The embryoid bodies then float freely in the culture medium. Each embryoid body consists of an unpredictable mix of partially differentiated cell types, but allowing the embryoid bodies to form is the most consistent way of allowing EG-derived cells to differentiate [11]. The process involves several stages of cell derivation in a different kinds of growth media. Cells from low-serum cultures were passaged, chemically dissociated, and resuspended in a culture media that contains 50% fetal bovine serum, and frozen in this state. To measure proliferation, cultures are derived from the frozen embryoid bodies and grown in the same media used to grow the dissociated cells. Clonal cell lines are then derived from the embryoid body-derived cultures [32].
The embryoid body-derived cells resulting from this process have high proliferative capacity and gene expression patterns that are representative of multiple cell lineages. This suggests that the embryoid body-derived cells are progenitor or precursor cells for a variety of differentiated cell types [11].
Recently, Neil Hanley and David Wilson from the University of Southampton, United Kingdom, have derived EG cells from the primordial germ cells of the fetal gonadal ridge. Using material at 8–10 weeks gestation, cells were derived slightly differently form the methods of Shamblott et al using a combination of irradiated fibroblast feeder layers and gelatin coated tissue culture dishes [12]. This method and the further characterization of the alkaline phosphatase/SSEA1-positive EG cells currently remains unpublished.
Directed Differentiation of Human Embryonic Stem Cells and Embryonic Germ Cells In Vitro
As with cultures of mouse ES cells, human ES cells begin to differentiate if they are removed from feeder layers and grown in suspension culture on a non-adherent surface. The human ES cells form embryoid bodies which, in the early stages, may be simple or cystic and filled with fluid. Although human embryoid bodies vary in their cellular content, many include cells that look like neurons and heart muscle cells [14, 25, 26].
After the human embryoid bodies form, they can be dissociated and replated in monolayer cultures which are then exposed to specific growth factors that influence further cell differentiation. Some growth factors induce cell types that would normally be derived from ectoderm in the embryo; these include retinoic acid, epidermal growth factor (EGF), bone morphogenic protein 4 (BMP4), and basic fibroblast growth factor (bFGF). Other growth factors, such as activin-A and transforming growth factor–beta 1 (TGF-ß1) trigger the differentiation of mesodermally derived cells. Two other factors, hepatocyte growth factor (HGF) and nerve growth factor (NGF), promote differentiation into all three germ layers, including endoderm. When these eight growth factors were added individually to cell cultures derived from embryoid bodies (generated from the H9 line from Thomson's laboratory), the cells differentiated into 11 cell types that represented all three germ layers. The identify of the differentiated human embryoid body-derived cells was determined by their morphology, growth characteristics and expression of messenger RNA (mRNA) for specific markers [30] (see Figure A.6 Gene Transcription, Translation, and Protein Synthesis).
Human embryoid body-derived cells will differentiate spontaneously into many kinds of cells, without the addition of growth factors. However, the addition of one of a number of growth factors resulted in cultures that were more likely to be populated by only one or two types of differentiated cells, as measured by mRNA transcripts expressed by the cells. Human embryoid body-derived cultures treated with bFGF differentiated largely into epidermal epithelial cells that express keratin, a protein in skin. Cells in activin-A–treated cultures formed muscle cell-like syncytium—fused, multinucleated populations of similar cells—that express the enzyme muscle-specific enolase. And cultures treated with retinoic acid differentiated into cells that resemble neurons and express neurofilament H. However, the same growth factor typically induced the expression of multiple markers; none of the resulting cell populations was homogeneous [30].
Spontaneous differentiation of human ES cells into hematopoietic cells, which form all the lineages of blood cells, is rare in vitro. However, by co-culturing human ES cells with mouse bone marrow stromal cells (irradiated to prevent their replication) in growth medium that contains fetal bovine serum, but no added growth factors, the cells differentiate to form what appear to be hematopoietic precursor cells. The partly differentiated cells express CD34, a marker for blood cell precursors. If these partly differentiated human ES cells are replated under conditions that allow them to form colonies of hematopoietic cells, they differentiate into erythroid cells, macrophages, granulocytes, and megakaryocytes [19] (see Chapter 5. Hematopoietic Stem Cells).
As indicated, human ES cells maintained in vitro have tendency to differentiate spontaneously, a characteristic that may not always be desirable. Thus, it may be necessary to devise methods that allow undifferentiated ES cells be selected from a culture that contains a mixture of differentiated, partially differentiated, and undifferentiated cells types. The undifferentiated ES cells could then be used for the purposes of directed differentiation, or they could be removed from cultures in which the differentiated cell types are the desired product. In either case, a suggested method for identifying undifferentiated ES cells is to introduce a marker gene—such as that encoding green fluorescence protein (GFP)—whose expression is driven by a gene that is specifically expressed in proliferating, undifferentiated cells, such as Rex1. Then, undifferentiated cells that express GFP can be selectively removed from human ES cultures by using a fluorescence activated cells sorter (FACS) [9] (see Appendix E.i. Markers: How Do Researchers Use Them to Identify Stem Cells?).
Joseph Itskovitz-Eldor and his colleagues are trying to direct the differentiation of human ES cells into cardiac myocytes. They use several of the human ES lines generated in James Thomson's laboratory. They report a number of cells in embryoid bodies that have contractile activity and express genetic markers consistent commonly found in cardiac myocytes [16] (see Chapter 9. Can Stem Cells Repair a Damaged Heart?). Karl Skorecki and his collaborators have had success in directing the differentiation of human ES cell lines (originating from the Thomson laboratory derivation) into pancreatic islet-like cells that secrete insulin. They have also reported the expression of insulin genes found in islet-like cells of the pancreas [6] (see Chapter 7. Stem Cells and Diabetes).
A new report indicates that it may be possible to direct the differentiation of human EG cells into neuronal cells that may play a role in restoring some function to paralyzed animals. The SDEC human cell line in this study was generated from embryoid bodies that formed in culture by the aggregation of human EG cells, and is referred to as an embryoid-derived cell line. SDEC cells express a panel of neuronal markers that include nestin, neurofilament, tau protein, neuron-specific enolase; the cells also express the glial cells markers glial fibrillary acidic protein, galactocerebroside, and CNPase. No in vitro assays that indicate cell function have been reported for SDEC cell assays. However, when SDEC cells were injected into the central canal of the spinal cord of rats—whose hind limbs were paralyzed by an induced form of amytrophic lateral sclerosis (ALS, also known as Lou Gehrig's disease)—the majority of animals showed some functional recovery. It is not clear whether the human embryoid body-derived cells replaced some of the spinal motor neurons damaged by the experimental ALS, or whether the injected cells triggered neurons in the recipient animals to recover lost function [17] (see Chapter 8. Rebuilding the Nervous System with Stem Cells).
Several groups of investigators are trying to direct the differentiation of human ES cell lines, but their work is not yet published. They reported their findings in interviews with the NIH or during presentations at scientific meetings. They include, but are not limited to, the following:
- Martin Pera, Alan Trounson, and their coworkers are trying to direct the differentiation of human ES cells along a neural lineage using the BMP antagonist noggin. They generate an apparently homogenous population of cells, but have not yet characterized it [23].
- Brenda Kahan, Jon Odorico, and their coworkers are trying to direct the differentiation of the human ES lines H1 and H9 into pancreatic islet cells, which are endodermal derivatives. They induce the formation of embryoid bodies in medium lacking bFGF and assay cultures for the expression of transcripts for the endodermal markers hnf3, lfabp, ifabp, and villin 1. Differentiated progeny from these cells express the genes for insulin, glucagon, and somatostatin, which are normally expressed in pancreatic islet cells [15].
- Micha Drukker, Nissim Benvenisty, and their colleagues are trying to direct the differentiation of human ES cells into neurons by adding retinoic acid or ß-NGF to the growth medium. They report that about 80% of embryoid bodies exposed to these factors contain differentiated neuronal cells, as determined by morphology and the expression of receptors for dopamine or serotonin [8].
- Su-Chang Zhang, James Thomson, and their collaborators are trying to direct the differentiation of human ES cells into neural epithelial cells, by selecting cells from embryoid bodies that express nestin, glial fibrillary acidic protein (GFAP, an astrocyte marker), neural cell adhesion molecule (NCAM), and Musashi-1. The differentiated cell types express (as yet unidentified) markers of neurons and glial cells. After transplantation into the mouse brain, the cells aggregated into clusters and migrate into the brain parenchyma where they express (unidentified) neural and glial markers. By 10 weeks after transplantation, the human embryoid-derived cells had not formed teratomas [38].
- Margaret Inokuma, Melissa Carpenter, and their colleagues are trying to direct the differentiation of human ES cells into neural cells using neurotrophin 3 (NT3) and brain-derived neurotrophic factor (BNDF). Some of the resulting cells stain positive for tyrosine hydroxylase (TH, the rate-limiting enzyme in dopamine synthesis) or gamma-amino butyric acid (GABA), an inhibitory neurotransmitter [13].
- Chunhui Xu, Melissa Carpenter, and their colleagues report preliminary data on growing human ES cells in vitro in serum-free medium without feeder layers. The details of their method have not been published but apparently include Matrigel or laminin as a substrate, basic FGF, and conditioned medium from cultures of mouse embryo fibroblasts [39].
- J.S. Lebkowski and Margaret Inokuma et al. report methods for using genetic modification and changes in culture conditions to direct the differentiation of the human ES cell lines H1 and H7 in vitro. They grow undifferentiated human ES cells in serum-free medium on Matrigel or laminin, and then add 20% serum replacement medium plus DMSO to direct the first stage of differentiation. The second stage is induced by adding sodium butyrate to the medium. Cell maturation occurs in a third medium (not described). To induce differentiation into neural cells, they allow the human ES cells to form embryoid bodies, which are then expanded, and plated in B27 medium (not described), with FGF and EGF. The resulting cells express the neural progenitor markers psNCAM and A2B5. Some differentiated cells express the glial marker GFAP. Other cells express the neuronal markers β-tubulin III and synaptophysin, or stain for the neurotransmitters GABA, tyrosine hydroxylase, or glutamate. No quantitative data, electrophysiological data, or responses to neurotransmitter application are reported [18].
Human Embryonic Carcinoma Cells
Embryonal carcinoma (EC) cells are the "stem cells" that occur in unusual germ cell tumors, also called teratocarcinomas. As such, they give rise to the differentiated cell types that also occur in the tumors. The tumors probably arise from a malignant form of a primordial germ cell. In humans, germ cell tumors occur most often in the testis of young men; these are always malignant, but usually treatable. Benign germ tumors called ovarian cysts can occur in the ovary; malignant ovarian germ cell tumors are much rarer (malignant ovarian tumors—usually referred to as ovarian cancer—are not germ cell tumors).
Germ cell tumors have been studied extensively in humans and mice. They contain an aberrant mix of differentiated cell types, rather than a single kind of tumor cell. Small groups of the cells may appear organized, but overall, the tissue in the tumor is disorganized. Teratocarcinomas are of particular interest because they contain EC cells, which in many ways resemble normal ES cells [4].
Like human ES cells, human EC cells proliferate extensively in vitro and in teratomas formed in vivo after injection into immunocompromised mice. Because research on human ES cells is so recent, a direct comparison of cultured human EC cells and human ES cells has just begun.2 Both cell types express a panel of surface markers, including the embryonic stage-specific antigens SSEA-3 and SSEA-4. Neither human ES cells nor human EC cells expresses SSEA-1, as mouse ES and EC cells do [5, 26, 35]. Conversely, mouse EC and ES cells do not express SSEA-3 or SSEA-4. Human EC and ES cells also carry on their surfaces keratin sulfate proteoglycans that can be labeled with specific antibodies, TRA-1–60 and TRA-1–81 [3, 7]. Also, unlike their mouse counterparts, human ES and EC cells express MHC Class I antigens, which are responsible for immunogenicity (see Chapter 6. Autoimmune Diseases and the Promise of Stem Cell-Based Therapies). Like mouse ES and EC cells, undifferentiated human ES and EC cells strongly express the transcription factor Oct-4 [26, 4], which is widely regarded as a hallmark of pluripotent embryonic cells [20, 28, 29] (see Table C.1. Comparison of Mouse, Monkey, and Human Pluripotent Stem Cells).
2 As of May 9, 2001, the comparisons between human ES cells and EC cells have been made only in Peter Andrews' laboratory at the University of Sheffield, United Kingdom. James Thomson, of the University of Wisconsin at Madison, supplied Andrews with four lines of human ES cells [
3,
32].
Human ES and EC cells differ in important ways. Human ES cells are euploid, meaning they carry the normal complement of chromosomes. In contrast, human EC cells are aneuploid; their chromosomes are distinctly abnormal. (Interestingly, the chromosomes in mouse EC cells do not appear as abnormal, although they do carry subtle chromosomal abnormalities.) The ability of both cell types to differentiate into various tissue types has been explored by injecting human ES and EC cells into immunocompromised mice. Injected human ES cells will form embryonic stem cell teratomas in mice, and the tumors consist of cells derived from all three primary germ layers [36]. In contrast, human EC cell lines vary in their ability to differentiate in vivo, but in general are more limited than are ES cells. For example, NTERA2 cl.D1 cells (which are derived from human TERA2 EC cells) generate only a few kinds of tissues, including primitive gut-like structures, and neural tissue after injection into immunocompromised mice [2].
Table C.1. Comparison of Mouse, Monkey, and Human Pluripotent Stem Cells
Marker
Name |
Mouse EC/
ES/EG cells |
Monkey
ES cells |
Human
ES cells |
Human
EG cells |
Human
EC cells |
| SSEA-1 |
+ |
– |
– |
+ |
– |
| SSEA-3 |
– |
+ |
+ |
+ |
+ |
| SEA-4 |
– |
+ |
+ |
+ |
+ |
| TRA-1–60 |
– |
+ |
+ |
+ |
+ |
| TRA-1–81 |
– |
+ |
+ |
+ |
+ |
| Alkaline phosphatase |
+ |
+ |
+ |
+ |
+ |
| Oct-4 |
+ |
+ |
+ |
Unknown |
+ |
| Telomerase activity |
+ ES, EC |
Unknown |
+ |
Unknown |
+ |
| Feeder-cell dependent |
ES, EG, some EC |
Yes |
Yes |
Yes |
Some; relatively low clonal efficiency |
| Factors which aid in stem cell self-renewal |
LIF and other factors that act through gp130 receptor and can substitute for feeder layer |
Co-culture with feeder cells; other promoting factors have not been identified |
Feeder cells + serum; feeder layer + serum-free medium + bFGF |
LIF, bFGF, forskolin |
Unknown; low proliferative capacity |
| Growth characteristics in vitro |
Form tight, rounded, multi-layer clumps; can form EBs |
Form flat, loose aggregates; can form EBs |
Form flat, loose aggregates; can form EBs |
Form rounded, multi-layer clumps; can form EBs |
Form flat, loose aggregates; can form EBs |
| Teratoma formation in vivo |
+ |
+ |
+ |
– |
+ |
| Chimera formation |
+ |
Unknown |
+ |
– |
+ |
Key
ES cell = Embryonic stem cell
EG cell = Embryonic germ cell
EC cell = Embryonal carcinoma cell
SSEA = Stage-specific embryonic antigen |
TRA = Tumor rejection antigen-1
LIF = Leukemia inhibitory factor
bFGF = Basic fibroblast growth factor
EB = Embryoid bodies |
The in vitro growth characteristics of human ES and EC cells are also being compared. Both cell types grow well in serum-containing medium on feeder layers of mouse embryonic fibroblasts that have been treated to block their proliferation. It is difficult to induce human ES cells to proliferate in the absence of feeder layers, unless conditioned medium from feeder cells cultures is added. However, many human EC cells lines, such as the NTERA2 line, are not dependent on feeder layers [2].
If human ES cells are removed from their feeder layers, they differentiate spontaneously into many cell types. Mouse ES cells, after removal from feeder layers, can be stimulated to divide and prevented from differentiating by adding LIF (leukemia inhibitory factor); neither human ES nor EC cells show this response to LIF. Instead, if human ES cells grow to confluence (where the cells grow to completely cover the culture plates), the cells aggregate and begin to differentiate spontaneously [26, 35]. Also, human ES cells grown in suspension cultures at high density will form embryoid bodies. Embryoid bodies are clumps or groupings of cells that form when cultured in plates or media and do not occur in nature. Embryoid bodies contain undifferentiated and partially differentiated cells [14]. However, human EC cells remain undifferentiated when grown at high density [4]. Whether these apparent differences in the in vitro growth characteristics of human ES and EC cells are meaningful or real is subject to debate [5].
The pluripotency of human EC cells does not equal that of human ES cells. Human ES cells can differentiate into a wide range of cell types in vitro, and can form teratomas with many cell types after injection into immune-deficient mice. The differentiation potential of most lines of human EC cells is more limited, both in vitro and in vivo. One human EC cell line, however, TERA2, differentiates easily in vitro. The well-studied morphogen, retinoic acid, induces TERA2 cells (and the subline NTERA2) to differentiate into neural precursors, which can then become mature neurons [4]. But when human ES cells are exposed to retinoic acid, they differentiate into a wider array of cell types than do human EC cells. As yet, it is not clear how the mechanism of action of retinoic acid differs in human ES cells versus human EC cells. It may be that, because of their tumor origin, human EC cells carry genetic variations linked to tumorigenesis that restrict their capacity for differentiation [5].
Thus, the in vitro and in vivo characteristics of human EC cells resembles that of human ES cells in certain respects, but not in others. Although ES cells will likely prove to be a better model for understanding human development than will EC cells [27], there may be some aspects of development that EC cells will reveal that ES cells will not [5].
References
- Amit, M., Carpenter, M.K., Inokuma, M.S., Chiu, C.P., Harris, C.P., Waknitz, M.A., Itskovitz-Eldor, J., and Thomson, J.A. (2000). Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271–278.
- Andrews, P.W., Damjanov, I., Simon, D., Banting, G.S., Carlin, C., Dracopoli, N.C., and Fogh, J. (1984). Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab. Invest. 50, 147–162.
- Andrews, P.W., Casper, J., Damjanov, I., Duggan-Keen, M., Giwercman, A., Hata, J., von Keitz, A., Looijenga, L.H., Millan, J.L., Oosterhuis, J.W., Pera, M., Sawada, M., Schmoll, H.J., Skakkebaek, N.E., van Putten, W., and Stern, P. (1996). Comparative analysis of cell surface antigens expressed by cell lines derived from human germ cell tumours. Int. J. Cancer. 66, 806–816.
- Andrews, P.W. (1998). Teratocarcinomas and human embryology: pluripotent human EC cell lines. Review article. APMIS. 106, 158–167.
- Andrews, P.W., personal communication.
- Assady, S., Maor, G., Amit, M., Itskovitz-Eldor, J., Skorecki, K.L., and Tzukerman, M. (2001). Insulin production by human embryonic stem cells. Diabetes. 50.
- Badcock, G., Pigott, C., Goepel, J., and Andrews, P.W. (1999). The human embryonal carcinoma marker antigen TRA-1–60 is a sialylated keratan sulfate proteoglycan. Cancer Res. 59, 4715–4719.
- Drukker, M., Schuldiner, M., Eiges, R., Eden, A., Yanuka, O., Itskovitz-Eldor, J., and Benvenisty, N. Keystone symposia. Pluripotent stem cells: biology and applications. Induced neuronal differentiation of human embryonic stem cells. Poster abstracts. 207.
- Eiges, R., Schuldiner, M., Drukker, M., Yanuka, O., Itskovitz-Eldor, J., and Benvenisty, N. (2001). Establishment of human embryonic stem cell-transduced clones carrying a marker of undifferentiated cells. Curr. Biol. 11, 514–518.
- Firpo, M., personal communication.
- Gearhart, J., personal communication.
- Hanley, N., personal communication.
- Inokuma, M.S., Denham, J., Mujtaba, T., Rao, M., and Carpenter, M.K. Keystone symposia. Pluripotent stem cells: biology and applications. Human embryonic stem cells differentiate into neural cells in vitro. Poster abstracts. 312.
- Itskovitz-Eldor, J., Schuldiner, M., Karsenti, D., Eden, A., Yanuka, O., Amit, M., Soreq, H., and Benvenisty, N. (2000). Differentiation of human embryonic stem cells into embryoid bodies comprising the three embryonic germ layers. Mol. Med. 6, 88–95.
- Kahan, B.W., Jacobson, L.M., Hullett, D.A., Thomson, J., and Odorico, J.S. Keystone symposia. Pluripotent stem cells: biology and applications. In vitro differentiation of human embryonic stem (ES) cell lines: expression of endoderm-and pancreatic islet-specific genes. Poster abstract. 117.
- Kehat, I., Kenyagin-Karsenti, D., Druckmann, M., Segev, H., Amit, M., Gepstein, A., Livne, E., Binah, O., Itskovitz-Eldor, J., and Gepstein, L. (2001). Human embryonic stem cells can differentiate into myocytes portraying cardiomyocytic structural and functional properties. J. Clin. Invest. (In press)
- Kerr, D.A., Llado, J., Shamblott, M., Maragakis, N., Irani, D.N., Dike, S., Sappington, A., Gearhart, J., and Rothstein, J. (2001). Human embryonic germ cell derivatives facillitate motor recovery of rats with diffuse motor neuron injury.
- Lebkowski, J.S., Gold, J., Chiu, C.P., Xu, C., Inokuma, M., Hassanipour, M., Denham, J., Piderit, A., Rosler, E., Golds, K., and Carpenter, M. ASGT Meeting. Targeted Gene Expression I. Human Embryonic Stem Cells: Genetic Modification and Differentiation into Cell Types for Potential Transplantation Applications. Poster Abstract. 205.
- Odorico, J.S., Kaufman, D.S., and Thomson, J.A. (2001). Multilineage Differentiation from Human Embryonic Stem Cell Lines. Stem Cells. 19, 193–204.
- Okamoto, K., Okazawa, H., Okuda, A., Sakai, M., Muramatsu, M., and Hamada, H. (1990). A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 60, 461–472.
- Panicker, M., personal communication.
- Pera, M., personal communication.
- Rao, M., personal communication.
- Reubinoff BE, Pera, M., Fong, C.Y., and Trounson, A. (2000). Research Errata. Nat. Biotechnol. 18, 559.
- Reubinoff, B.E., Pera, M.F., Fong, C.Y., Trounson, A., and Bongso, A. (2000). Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 18, 399–404.
- Roach, S., Cooper, S., Bennett, W., and Pera, M.F. (1993). Cultured cell lines from human teratomas: windows into tumour growth and differentiation and early human development. Eur. Urol. 23, 82–87.
- Rosner, J.L. (1990). Reflections of science as a product. Nature. 345, 108.
- Schöler, H.R., Ruppert, S., Suzuki, N., Chowdhury, K., and Gruss, P. (1990). New type of POU domain in germ line-specific protein Oct-4. Nature. 344, 435–439.
- Schuldiner, M., Yanuka, O., Itskovitz-Eldor, J., Melton, D., and Benvenisty, N. (2000). Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 97, 11307–11312.
- Shamblott, M.J., Axelman, J., Wang, S., Bugg, E.M., Littlefield, J.W., Donovan, P.J., Blumenthal, P.D., Huggins, G.R., and Gearhart, J.D. (1998). Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. U. S. A. 95, 13726–13731.
- Shamblott, M.J., Axelman, J., Littlefield, J.W., Blumenthal, P.D., Huggins, G.R., Cui, Y., Cheng, L., and Gearhart, J.D. (2001). Human embryonic germ cell derivatives express a broad range of develpmentally distinct markers and proliferate extensively in vitro. Proc. Natl. Acad. Sci. U. S. A. 98, 113–118.
- Thomson, J., personal communication.
- Thomson, J.A., Kalishman, J., Golos, T.G., Durning, M., Harris, C.P., Becker, R.A., and Hearn, J.P. (1995). Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. U. S. A. 92, 7844–7848.
- Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S., and Jones, J.M. (1998). Embryonic stem cell lines derived from human blastocysts. Science. 282, 1145–1147.
- Thomson, J.A. and Odorico, J.S. (2000). Human embryonic stem cell and embryonic germ cell lines. Trends. Biotechnol. 18, 53–57.
- Totey, S., personal communication.
- Xu, C., Inokuma, M.S., Denham, J., Golds, K., Kundu, P., Gold, J.D., and Carpenter, M.K. Keystone symposia. Pluripotent stem cells: biology and applications. Growth of undifferentiated human embryonic stem cells on defined matrices with conditioned medium. Poster abstract. 133.
- Zhang, S.U., Wernig, M., Duncan, I.D., Brüstle, O., and Thomson, J. Keystone symposia. Pluripotent stem cells: biology and applications. Directed differentiation of human ES cells to neural epithelia. Poster abstracts. 235.
 Appendix B | Table of Contents | Appendix D
Appendix B | Table of Contents | Appendix D 
Historical content: June 17, 2001