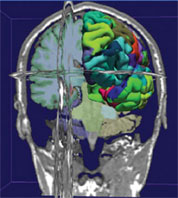
The NCRR-supported Morphometry Biomedical Informatics Research Network (mBIRN) is pooling and analyzing data across neuroimaging sites to explore potential relationships between anatomical differences and specific dysfunctions of memory. This image of brain morphology reveals segmentation of white and gray matter and cortical parcellations generated by the FreeSurfer software tool and interactive visualization by 3-D Slicer. The data were collected by mBIRN as part of a multisite, multivendor magnetic resonance calibration effort to improve the accuracy and statistical power of large-scale brain imaging clinical studies. (Photo Credit: Steve Pieper, Isomics, Inc., and Surgical Planning Laboratory, Brigham and Women’s Hospital)
Informatics is an overarching theme that permeates all the strategies and areas in this Plan—clinical and translational research, animal models, technology development, and advancing underserved communities. All investigators require new informatics knowledge and tools that will allow them to:
NCRR is committed to working with other agencies, NIH ICs, and industry to bring informatics knowledge and tools to researchers at all levels. Several NCRR-supported programs currently provide informatics resources.
NCRR will continue to support and address integration of informatics research and solutions in all its programs and centers. In addition, NCRR will pursue integration across the various domains of knowledge and research within NCRR and its partners at other NIH ICs, other federal agencies, industry, and foundations. Issues of integrity, durability, availability, and security of data will continue to play a critical role in this era of fast-moving technologies and analysis tools.

In 2006, Vanderbilt University used institutional resources to establish a DNA specimen and databank repository that is available to all Vanderbilt and Meharry Medical College investigators. Informatics support for requests to retrieve, genotype, and analyze these biobank samples and their associated de-identified clinical information will be provided through competitive funding requests from Vanderbilt University’s CTSA program. This repository is a potent example of cross-program NCRR collaboration, given that Meharry Medical College has been a long-standing recipient of RCMI funding to support the infrastructure of shared resources. This image depicts a scientist in a laboratory logging samples in test tubes into a computer database; the inset shows the barcode scanning system used for cataloging biological samples. (Photo Credit: Dana C. Johnson, Vanderbilt University Medical Center)
Connectivity to the Web and wide access to informatics tools enable information sharing and communication across geography and disciplines, as Google and other informatics resources have clearly shown. PubMed Central will make the results of NIH-supported published biomedical research widely available. Sharing of raw data has become commonplace in some fields, including the human genome sequence, as well as those of many other species. Other genetic and phenotype data are being collected and made available through the National Library of Medicine. Additionally, many NIH ICs, other federal agencies, and private organizations make data available for research. Many tools for data analysis also are broadly and freely available from NIH, investigators, and organizations. However, there are areas for progress in data availability and, in particular, in sharing of metadata associated with the data (i.e., data that increase the usability and quality of the data).
Sharing of de-identified raw clinical data and clinical research data is also common, including from the Centers for Medicare and Medicaid Services, NIH studies, and foundations. Careful attention is required to assure privacy and confidentiality in sharing and use of human data for research. Differing and conflicting regulations and approaches have made sharing of clinical data more difficult.
Many challenges still exist to facilitate information sharing for biomedical research:

University of Delaware researchers collaborate with physicians at the Christiana Care Health System to develop innovative methods for visualizing complex biomedical data. This image, created by Karl V. Steiner, Ph.D., at the Delaware Biotechnology Institute, shows work based on a study conducted by Thomas Bauer, M.D., a thoracic surgeon at the Helen F. Graham Cancer Center, as part of the “International Early Lung Cancer Action Program (I–ELCAP).” The data are displayed using Starlight, a visualization-based information system developed by Battelle that allows for interactive, visual analysis of epidemiology patterns. The image displays multivariant correlations of such topics as patient disease history, diagnosis, age, gender, body mass index, home location, and smoking patterns. The project was supported, in part, by NCRR through the Delaware INBRE program. (Photo Credit: University of Delaware)
Action Items: NCRR will:
The transformation of clinical and translational science requires a visionary approach to management and sharing of information, which can only be accomplished with ubiquitous access to tools and processes by investigators to enhance the quality, availability, security, collection, and analysis of data. It is recognized that the informatics needs identified today for clinical and translational research will be rapidly augmented by new demands. Therefore, the supporting infrastructure needs to be flexible to respond to new challenges as they arise and scalable to accommodate increases in demand and the amount of data.
The performance of clinical and translational research requires a systematic approach to defining hypotheses and selecting a study design, including, in many cases, appropriate statistical power, that assures the study questions can be answered without putting human participants at inappropriate risk. In addition, clinical research requires multiple reviews by regulatory bodies and, in some cases, oversight by a data and safety monitoring board. This process can be complicated and may require multiple revisions of the protocol prior to implementation.
At many institutions, investigators do not have tools to assist in this process nor in the performance of the study. Informatics-based approaches have been very helpful in tracking protocol development and approvals and also in assuring that the quality of the study and its ability to be implemented are not impeded in the process.
Action Items: NCRR will:
Significant deficiencies exist in the approaches to support clinical studies and trials at academic institutions. Process and workflow modeling of clinical study development—from hypothesis formation through protocol and informed consent development; regulatory approvals; study implementation, including data collection; case report form design; data validation; analysis; and administrative and budgetary management—should facilitate the design and utilization of novel approaches. Informatics-based approaches grounded in the processes and workflows of clinical researchers and those involved in research with human participants will increase safety, quality, and ability to efficiently conduct such studies at academic institutions.
Action Items: NCRR will:
The challenges of conducting biomedical research currently and in the future require that researchers have an understanding of a set of core competencies in informatics. While many informatics training programs exist in the country, supported by the National Library of Medicine, there are few training programs in clinical and translational science and veterinary medicine that include informatics as a part of their curricula. However, these scientists will need to have a working knowledge of basic concepts regarding standards, ontologies, database theory, and knowledge management to use the tools and approaches required to design, conduct, and analyze research projects. The challenge is to increase the number of “multicapable” scientists working at the interface between basic science and the basic knowledge that comes from new technologies; having scientists who can think about this translates into emerging clinical opportunities.
Action Items: NCRR will:
To establish effective collaborations and partnerships and use the most effective tools, researchers must have access to and knowledge of state-of-the-art resources, technologies, and people in relevant areas. Many online resource and collaboration networks are arising, driven by this need. However, information about many NIH-supported resources is fragmented and difficult to locate, even via Internet searches. NCRR provides many resources in its multiple programs that could be further utilized.
Action Item: NCRR will pursue the development of a Web-based knowledge
community of NCRR resources that encourages access by all biomedical researchers.
NCRR will explore tools that allow users to interactively query the resources and
community, analyze spatial information, and explore relationships.