|
Frequently Asked Questions for the NHLBI Pediatric Cardiovascular Translation Consortium

General Information
Q: Which RFAs are part of the NHLBI Pediatric Cardiovascular Translation Consortium?
A: The RFAs are:
Q: Will potential applicants have the opportunity to meet with NHLBI staff to ask questions and obtain advice?
A: Yes, electronic informational meetings are scheduled October 31 and November 20 at 1-3pm EST.
- The meeting will start with a short introduction to the Pediatric Cardiovascular Translation Consortium by NHLBI staff, followed by a question and answer session.
- Questions can be submitted by:
- Emailing them in advance to the Project Officer listed at the end of the FAQ list.
- Or, using the chat feature to send questions to the meeting host during the meeting.
- To join the meeting, log in at https://webmeeting.nih.gov/NHLBIptc . Click on the radio button “Enter as Guest”, and a box will open for you to enter your name. Names are visible to other participants when you submit a question, so if you want to remain anonymous, type “Guest” in the name box.
- To make sure your computer configuration will support your participation in this Adobe Connect meeting, you can test your system prior to the meeting by going to http://webcollaboration.nih.gov/systemRequirements.asp. Audio may be transmitted through the internet link, so participants will need computer speakers to hear the meeting. Closed captioning will be provided.
- The meetings will be recorded. The October 31 recording is available at https://webmeeting.nih.gov/p89458785/
NHLBI staff will also respond to questions submitted by potential applicants who contact the program, grants management, and review staff listed in the RFAs in the Agency Contacts section.
Q: What is the NHLBI’s overall vision for pediatric cardiovascular translational research, and how does the Pediatric Cardiovascular Translation Consortium fit within this vision?
A: Click for a larger PDF version of chart or description of chart.
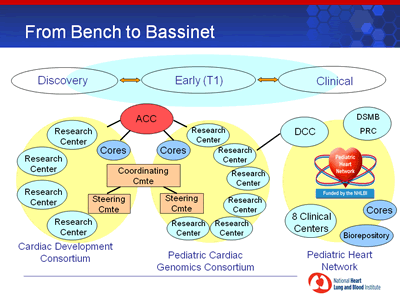
The NHLBI translational program “From Bench to Bassinet” will align the new Consortia with an existing clinical research network, the Pediatric Heart Network.
- The Cardiac Development Consortium will consist of up to four Research Centers, Cores, and a Steering Committee, and aims to link academic research groups together with shared vision, approach, and resources to fill in the gaps in understanding cardiovascular transcriptional networks and their interactions with cellular and tissue processes in animal models .
- The Pediatric Cardiac Genomics Consortium will consist of up to six Research Centers, Cores, and a Steering Committee. Each Research Center will focus on the investigation of the genetics and genomics of one or more common human cardiac malformations, to identify not only causative genes, but also genetic influences on outcome. The Consortium will coordinate patient recruitment across the multiple sites. The Pediatric Cardiac Genomics Consortium will also interact with the Pediatric Heart Network through the existing network Data Coordinating Center. The Network consists of eight Clinical Centers, Cores, and a biorespository, and has oversight by a Data and Safety Monitoring Board and a Protocol Review Committee.
- An Administrative Coordinating Center will handle the administrative functions of the two consortia, such as organizing meetings and calls of the Steering Committees, Coordinating Committee, External Advisory Panel, and Consortia joint annual meeting; designing and maintaining written materials and websites to support both Consortia; and awarding subcontracts for Cores. The two Steering Committees will interact by forming a Coordinating Committee that will provide scientific coordination between the two Consortia.
Application Instructions
Q: What is meant by the term “applicant”, and can applicants submit more than one application?
A: The “applicant” is the grantee institution submitting the application. If there are sufficient resources at a single institution to justify multiple applications, an institution may submit multiple applications for the CDC and PCGC RFAs, but only one ACC application will be allowed from a single institution.
Q: Can an investigator participate on the research team of more than one application?
A: Yes, but it is generally not recommended that an investigator be part of two competing applications being reviewed by the same review panel.
Q: Will each investigator submit an individual application, or should research teams submit a combined research center application?
A: Each application will propose a Research Center, and each Center will consist of a research team with the appropriate expertise to accomplish the specific aims. Awarded Centers will establish collaborations within the consortium during the first year planning phase. This differs from the NHLBI Progenitor Cell Consortium program, which has a two-phase application process where individuals who are awarded R03 grants during the first phase will assemble research teams and submit Center applications during the second phase.
Q: Can a Research Center proposal overlap with an existing R01 (or other) award?
A: Yes. If the proposal overlaps with an existing award, the overlap should be acknowledged in the support section of the PI’s biosketch, and a statement should be included explaining how the overlap will be resolved. For example, it could say that the overlapping portion of the R01 will be relinquished if the U01 is awarded.
Q: Are there page limits for the descriptions of qualifications and experience and for collaboration that are described in the RFA Other Submission Requirements section?
A: These elements should be incorporated into the appropriate narrative sections of the application, and within the 30 pages limit of the Research Plan.
Q: Will the Research Centers be organized like Program Project Grants, with multiple subprojects?
A: No. The Research Centers will each propose a project or group of related projects consistent with the objectives of the RFA and organized as a single set of specific aims. In addition, because the funding mechanism for this program is a cooperative agreement (U01), and because of the overall design of the Consortium, significant collaboration across Centers will be expected. For additional details, see the “Research Plan” section in the RFA.
Q: Can the Research Centers have multiple PIs, and can the PIs be from more than one university/institute?
A: Yes on both counts. Applicants may use the multiple-PI option, and the PIs can be located at different sites. If an application proposes multiple PIs, the leadership plan must address how leadership duties will be shared, and the application should follow the PHS 398 instructions at http://grants.nih.gov/grants/funding/phs398/phs398.html regarding Consortium/Contractual arrangements, such as including separate detailed budget pages for the sub-awards.
Q: For multiple PI proposals, should the themes be separate as in multiple components of a program project or integrated into a single more R01-like research plan?
A: They should be integrated.
Q: The RFAs state that the applicant should have an established research program in the area of study. Does “applicant” refer to the PI, or the research team?
A: The “applicant” is the institution submitting the grant application. The PI or PIs are expected to have significant experience and expertise in the area of study, as are other key members of the research team. The applicant institution should currently support research in the area of study. The combination should ensure the highest likelihood of consortium success.
Cores
Q: How does the purpose of consortium Cores differ from those associated with PPGs or SCCORs, and how will their selection process differ?
A: The purpose of Cores within a consortium is to make services centrally available to consortium sites. This differs from a PPG or SCCOR Core that serves only the subprojects within each program or Center. PPG/SCCOR Cores are approved for funding based on the advice of the review panels, while consortium Cores will be selected by the NHLBI during the first-year planning phase, with input from the Steering Committees (composed of PIs and others; see the RFAs for specific membership) and an External Advisory Committee, based on the needs of the consortium and merits of the candidate Cores, and the funds available within the Consortium. The NHLBI anticipates that many proposals will need common Cores.
Q: Can one have center-specific Cores within Research Centers, such as for preparing DNA or an administrative Core?
A: We would not call it a Core if it is serving just one Research Center and is not available to the rest of the consortium. Services that must be provided locally, such as routine animal husbandry, should not be proposed as a Core, but should be included in the Center proposal and budget.
Q: How can an investigator know the capabilities of Cores to be supported by the consortium?
A: Since the Cores will not be selected until during the planning year, investigators will not know the specific capabilities of the Cores at the time of application. The applications instead should describe the Core services needed and expect that the services will be available through the consortium if the application is funded. Keep in mind that the principal investigators will serve on the Steering Committees and will have a role in determining which Cores that consortia require.
Q: Will the Cores be in one geographical place?
A: No. The Cores may be located at different geographic sites, as long as they are capable of serving the needs of the consortium.
Q: If a Core is required for a given Research Center proposal, can the Center expect that the Core will be located at the Center?
A: Not necessarily. If multiple Centers propose the same type of Core, only a single Core site will be selected. The selected Core will be chosen based on its ability to serve the entire consortium, and may not necessarily be located at a Research Center.
Q: Is a detailed description of the function and organization of the Cores required in addition to a budget for the core?
A: Yes. A description of the services to be provided by the Core should be included in the Research Plan, which is part of the reason for the increased page limit. The organizational details may better fit in the budget justification section of the Core budget pages.
Q: How will the Core funds be administered?
A: The ACC will receive funds specifically designated for distribution to Cores, separate from the ACC operating funds, and will distribute the funds to the Cores via subcontracts.
Q: What will happen to a Research Center if they propose a Core that would provide the Center with certain scientific functions critical for the proposal, but the Core is not selected during the planning year?
A: The NHLBI can use the flexibility of a cooperative agreement to assure that any funded Research Center will have access to the resources and services necessary to complete the work as funded.
Cardiac Development Consortium
Q: How much preliminary data is expected for the CDC proposals?
A: The preliminary data should be sufficient for the reviewers to assess the likelihood of success and the value of the data generated. There should be evidence that the Research Center will be technically capable of generating the proposed data, but it is not expected that full data sets will be available preliminarily. High risk proposals will be weighed against their potential impact, so sufficient preliminary data to suggest high impact of the data output will increase the scientific merit of a proposal.
Q: How much of a mix of discovery-based science versus hypothesis-testing is envisioned for the CDC?
A: The purpose of the CDC is to support basic collaborative research leading to a comprehensive understanding of the regulatory networks controlling cardiovascular development. Research Center applicants will propose projects that will select key regulatory pathways, identify the components of the pathways and targets, and rapidly disseminate data to the scientific community. Therefore, data generation will be a major aspect of the consortium, but the consortium will also include limited hypothesis -testing as needed to test the usefulness of the data generated.
Q: It appears that an important focus for the CDC projects is to identify pathway members on a genome- wide level. Are studies into the function of individual pathway members appropriate?
A: No. Mechanistic studies of individual components within pathways are more appropriate as investigator-initiated R01 applications.
Q: Given that the Cardiac Development Consortium will attempt to coordinate goals, it appears that Centers may be included because they help to support goals of other Centers, rather than solely based on the individual potential of that Center's program. Won't that potentially diminish the scientific excellence of each project?
A: This is not correct. As with all consortia and networks, the merits of the proposal will be the primary review consideration. Consortium collaboration is expected and evidence of the potential for this will enhance the merit of the proposal. The goal is uniform scientific excellence of the consortium as a whole.
Q: What would the NHLBI consider as appropriate Cores for the CDC?
A: The NHLBI has not predetermined what Cores will be needed, but will consider Cores appropriate if they enhance the goals of the consortium. For example, the RFA emphasizes the importance of data interoperability between the Research Centers, so the RFA mentions a bioinformatics core as an example of a Core that might be appropriate.
Pediatric Cardiac Genomics Consortium
Q: Can a Research Center in the PCGC propose to study multiple malformations/syndromes?
A: A Research Center can propose to study multiple syndromes, but ideally, they will be functionally or anatomically related or have a common genetic or genomic theme.
Q: Is the PCGC limited to studying only cardiac malformations?
A: The main focus of research in the Consortia will be congenital cardiovascular malformations. However, studies of closely related topics, such as pulmonary vascular malformations associated with cardiac malformations, would be considered responsive to these RFAs.
Q: Should PCGC applications calculate sample size and statistical power based on the cohort at their Research Center, or all Centers in the consortium? Should the budget for patient-related costs reflect the number of patients for the proposed study, or for the available patient population at the Research Center?
A: Sample size and statistical power calculations should be based on the expected recruitment by the entire consortium. The study or studies proposed should be those that are scientifically meritorious and can benefit from multi-center recruitment to obtain sufficient study subjects. The sample size should be based on the scientific question to be answered, and be a realistic expectation given recruitment at 6 Centers. The budget for the patient-related protocol costs should reflect the total direct costs of implementing the proposal across ALL Research Centers, not just at the applicant’s Center. For further guidance about what to include in the protocol budget, please refer to the RFA.
Q: Can the costs of patient recruitment be shifted to a Core?
A: No. Some costs of protocol development and training may be assumed by the PHN DCC, but patient recruitment costs are to be included in the Research Center budget.
Q: What is the rationale for the nearly 2-fold difference in budgets for the CDC and PCGC Centers?
A: The CDC and PCGC have different primary goals. Our expectation is that the CDC will have a data generating function and rapidly disseminate the data. The CDC budget reflects the cost of state-of-the-art technologies that will push the envelope to generate data not currently available to the scientific community. The PCGC will function to coordinate patient recruitment, and though each Center has a lower budget than CDC Centers, the PCGC has a larger number of Centers than the CDC. The budgets for the CDC and PCGC were developed based on the studies expected in each consortium and the resources required to conduct the studies. Additionally, PCGC resources will be leveraged by interaction with the PHN Cores, DCC, and biorepository.
Q: Will there be a genotyping core for the PCGC? If so, will it likely be housed in an NIH-funded sequencing center?
A: Not necessarily, but since genotyping will likely be a required service useful to multiple Research Centers, it might be a reasonable Core for applicants to describe in their applications. Selected Cores will be those that can provide the required service, and may or may not be associated with existing NIH-funded sequencing centers.
Q: Are there cores that are part of the Pediatric Heart Network that can be used as part of PCGC projects – such as cores that characterize clinical characteristics of patients to be genotyped?
A: It is NHLBI’s intent to leverage the resources of the PHN and the PCGC in mutually beneficial ways. Current PHN cores, such as imaging and biorepository cores, may be suitable for use by the PCGC. These decisions will be made in the planning year, and will depend on the amount of funds available in the PHN and the PCGC for these purposes.
Q: Do you imagine that PHN Centers will be contributing DNA to the new consortia, regardless of their participation as a Research Center in the PCGC?
A: As noted in the answer to the previous question, it is NHLBI’s intent to maximize efficiency in the PHN and the PCGC. It is possible that PHN centers that are not members of the PCGC might participate in PCGC protocols, and thus contribute both DNA and clinical information.
Planning year
Q: What will be accomplished during the first-year planning period?
A: The center PIs from each Consortium will form the Steering Committees and Coordinating Committee. The CDC Steering Committee will plan large experiments, assess needs for cores and propose cores, and develop standardized analysis platforms. The PCGC Steering Committee will develop protocols and related materials, determine how to coordinate patient recruitment, and identify needed core labs. The Coordinating Committee will assure communication between the two steering committees during the planning process, and throughout the project period. The ACC will organize committee conference calls and meetings and the annual meeting, construct Consortia websites, and design the necessary written materials to support the Consortia. NHLBI staff will be involved as both administrative and scientific collaborators in the Consortia, through the cooperative agreement mechanism.
Q: How closely will the projects and cores implemented during the first year planning year correlate with the proposals in the applications?
A: The proposals submitted by successful applicants will form the starting point for the discussions in the Steering and Coordinating Committees in the planning year. During these discussions, areas of shared interest will be identified among the Research Centers, along with new scientific opportunities that may have arisen since the grant applications were submitted. It is likely, therefore, that the scientific proposals will evolve in some ways during this process, but the main thrust of the proposed science is not likely to change dramatically. Please see the FAQ section on Cores for additional information.
Q: Sharing of DNA from patients will require IRB approval and consent. Will a sharing protocol be developed during the planning year?
A: Full clinical study protocols are expected and will be finalized for all PCGC studies during the planning year, with the assistance of NHLBI and the PHN Data Coordinating Center. Protocol materials will include consent form templates to be submitted as part of the IRB package at each site. Recruitment cannot begin at a site until IRB approval has been obtained. The protocols and consent forms will be standardized according to current policy and Good Clinical Practice principles.
Q: What are the appropriate uses of the $160K budget during the first year?
A: The budget for the planning year should include a minimum of 2.4 calendar months effort for the applicant PI or a minimum of 2.4 calendar months combined effort for all PIs if the multiple PI strategy is used; travel funds for two 2-day Steering Committee meetings in Bethesda, MD for the PI and coordinator, travel funds for the PI(s) to attend one Coordinating Committee meeting in Bethesda, MD, and travel funds for 2-3 days of study coordinator protocol training in Boston; a 0.5 FTE clinical study coordinator for PCGC Centers; effort for co-investigators active during the planning year, such as a bioinformatician; and additional items as needed.
Administrative Coordinating Center
Q: Will the ACC have a role in the research directions of the two consortia?
A: No. The ACC will cover administrative functions and distribute Core funds, but will not select the Cores and will not be the site of the Cores. The ACC PI will be expected to have expertise in administering large-scale research collaborations, but is not required to have scientific expertise in development or genomics. The PI of the ACC will, however, serve on the consortia Steering and Coordinating Committees.
Q: Who will write the ACC research plan?
A: The ACC PI is responsible for planning, directing, and executing the research plan, but since the ACC research plan will not be a scientific proposal, the plan should address the elements described in the RFA.
Q: Will the ACC be responsible for obtaining, or assisting in obtaining, IRB or OMB approvals?
A: The PHN Data Coordinating Center will have the main responsibility for putting together protocol packages to support IRB approvals. We’re not anticipating the need for OMB approvals, but if that were to be necessary, they could be handled the same way.
Q: How will the PHN Data Coordinating Center interact with the consortia?
A: NHLBI expects to leverage the resources of the current PHN DCC for functions that are common to the PHN and the PCGC. These include development of common case report forms, coordination of specimen collection and handling with the PHN biorepository, and training of PCGC study coordinators.
Project Officer:
Charlene Schramm, Ph.D.
Division of Heart and Vascular Diseases
National Heart, Lung, and Blood Institute
Rockledge 2, Room 8100
6701 Rockledge Drive
Bethesda, MD 20892-7940
Telephone: (301) 435-0510
E-mail: schrammc@nhlbi.nih.gov
Last updated: November 18, 2008
|