Appendix A: Early Development
How does a single cell—the fertilized egg—give rise to a complex, multicellular organism? The question reflects one of the greatest mysteries of life, and represents a fundamental challenge in developmental biology. As yet, knowledge about the processes by which a fertilized egg divides (cleavage), forms a ball of cells (morula), develops a cavity (blastocyst stage), forms the three primary germ layers of cells that will ultimately give rise to all the cell types of the body (gastrula stage), and ultimately generates all the specialized tissues and organs of a mature organism is far from complete. Little is known about the specific genes that regulate these early events or how interactions among cells or how cellular interactions with other factors in the three-dimensional environment of the early embryo affect development. The processes by which a fertilized egg becomes an embryo, called embryogenesis, include coordinated cell division, cell specialization, cell migration, and genetically programmed cell death [24, 35].
A description of the stages of early embryogenesis in humans and mice follows. It includes an explanation of some of the more technical terms and concepts that are used throughout the document. It also includes a selective discussion of some of the genes, molecules, signaling pathways, and other influences on early embryonic development in the living organism (in vivo) that are used in experiments with stem cells maintained in the laboratory (in vitro).
Experimental Systems Used to Understand Embryogenesis
Many kinds of experimental systems have been used to understand how a fertilized egg produces a blastocyst, the first structure in which any cell specialization occurs, and a gastrula, in which the three embryonic germ layers—endoderm, mesoderm, and ectoderm—first appear. They include experiments with yeast cells; invertebrates such as tiny, jellyfish-like hydra, the microscopic roundworm Caenorhabditis elegans, and the fruit fly Drosophila melanogaster; and vertebrates such as amphibians, chick embryos and more recently, zebrafish Danio rerio, which are transparent as embryos and allow the detailed monitoring of cell differentiation and migration during development. The vast majority of studies on embryogenesis in mammals has been conducted in mice. For obvious ethical reasons, detailed research on human embryos has been limited. But the study of embryogenesis in all of these systems yields as many questions as answers.
For example, what signals the earliest cell differentiation events in the embryo? What regulates the activity of genes that are important for embryonic development? How and when are the axes of the embryo's body—anterior-posterior (head-tail), dorsal-ventral (back-belly), left-right—determined? What role does genetically controlled cell death, also known as apoptosis, play in embryogenesis? What influences the cell cycle, the controlled series of molecular events that leads to cell division or the cessation of cell division?
Much of the information about human embryonic development comes from studies of embryogenesis in the mouse. Like mammalian embryonic development in general, many aspects of embryogenesis in mice resembles that of humans, but development in mice also differs in several important respects from human development. For example, embryonic and fetal development in mice takes 18 to 20 days; in humans, the process takes nine months. The placenta forms and functions differently in the two species. In humans, an embryonic disk develops after the embryo implants in the uterine wall, whereas in mice an egg cylinder forms. The yolk sac of a mouse embryo persists and functions throughout gestation; in humans, the yolk sac functions only in early embryogenesis [28]. The primary roles of the human embryonic yolk sac are to initiate hematopoiesis and help in the formation of the primary germ cells, which will ultimately differentiate into eggs and sperm in the adult. And even for mice, knowledge about the genes, factors, and signal-transduction pathways that control embryonic development is limited. Signal transduction is a series of molecular events triggered by a signal at the surface of the cell and leading to a response by the cell—the secretion of a hormone, or a change in the activity of a particular gene, for instance [20].
Other sources of information about human development include studies of human embryonal carcinoma (EC) cells maintained in vitro and of histological sections of human embryos. (EC cells are derived from unusual tumors called teratocarcinomas, which may form spontaneously in the human testis or ovary.) Also, within the past 20 years, clinics and research institutes in many countries have developed in vitro conditions that allow fertilization and blastocyst formation. Thus, the study of methods to improve pregnancy rates following in vitro fertilization (IVF) has yielded important information about early human embryogenesis.
A Fertilized Egg Forms a Blastocyst
Prior to fertilization in humans and mice, the egg (oocyte) enlarges, divides by meiosis, and matures in its ovarian follicle until it reaches a stage of meiotic division called metaphase II (see Figure A.1. Cell Cycle). At this point, the follicle releases the oocyte into the oviduct, one of two tube-like structures that lead from the ovaries to the uterus. The mature oocyte, a haploid cell that contains half the normal number of chromosomes, is surrounded by a protective coat of noncellular material (made of extracellular matrix and glycoproteins), called the zona pellucida. For fertilization to occur, a haploid sperm cell must bind to and penetrate the zona pellucida, fuse with the cell membrane of the oocyte, enter the oocyte cytoplasm, and fuse its pronucleus with the oocyte pronucleus. Fusion of the sperm and egg pronuclei restores the number of chromosomes that is typical of a given species. In humans, the normal diploid number of chromosomes for all the cells of the body (somatic cells) is 46 (23 pairs of chromosomes). Mature sperm and egg cells (germ cells) in contrast, carry only 23 chromosomes, the haploid number [1].
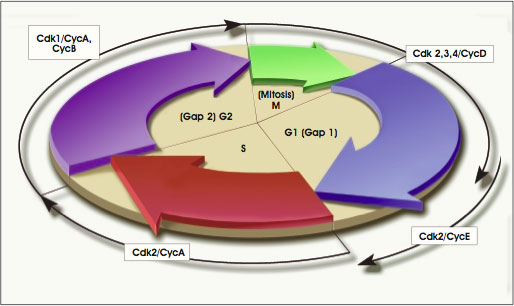
Figure A.1. Cell Cycle.
(© 2001 Terese Winslow, Lydia Kibiuk)
Under normal conditions, fertilization of the human oocyte occurs in the oviduct, near the ovary. A human egg is many times larger than a sperm cell, which means the oocyte contributes most of the cytoplasm of the zygote, another name for the fertilized egg. As a result, any maternal gene products in the zygote cytoplasm influence its first few divisions, called cleavages. Within several days, and after several cleavages, the genome (all the DNA or hereditary information in the cell's chromosomes) of the zygote becomes activated and controls subsequent embryonic development [19, 28]. Also, during these initial cleavages, the resulting daughter cells do not increase in size. Rather, as early cell division proceeds, the amount of cytoplasm of each daughter cell is reduced by half, and the total volume of the early embryo remains unchanged from that of the fertilized egg [30, 35].
After fertilization, the zygote makes its way to the uterus, a journey that takes three to four days in mice and five to seven days in humans. As it travels, the zygote divides. The first cleavage produces two identical cells and then divides again to produce four cells. If these cells separate, genetically identical embryos result, the basis of identical twinning. Usually, however, the cells remain together, dividing asynchronously to produce 8 cells, 16 cells, and so on [19]. Each early round of cell division takes approximately 36 hours, according to information gleaned from the study of human embryos in vitro [34]. In humans and mice, at about the eight-cell stage, the embryo compacts, meaning that the formerly "loose" ball of cells comes together in a tight array that is interconnected by gap junctions. These specialized membrane structures consist of an array of six protein molecules called connexins, which form a pore that allows the exchange of ions and small molecules between cells [27].
Recent information from studies of mouse embryos indicates that even at this early stage of embryogenesis, the anterior-posterior axis of the embryo has been established, a point of some concern for in vitro fertilization techniques, which disrupt early patterning events. The establishment of the anterior-posterior axis is critical to normal fetal development, because it helps determine the overall body plan of the embryo [3, 15, 18].
By the 16-cell stage, the compacted embryo is termed a morula. In mice, the first evidence that cells have become specialized occurs when the outer cells of the 16-cell morula divide to produce an outer rim of cells—the trophectoderm—and an inner core of cells, the inner cell mass [19]. Although the signals within the 16-cell morula that regulate the differentiation of the trophectoderm are largely unknown, it is clear that the outer cells of the morula are polarized. That is, one side of the cell differs from the other side. Thus, in the first differentiation event of embryogenesis, the outer, polar cells give rise to trophectoderm and the inner, apolar cells become the inner cell mass. This suggests that individual cells of the early embryo exhibit more intrinsic polarity than had been thought [27].
Ultimately, the cells of the inner cell mass will give rise to all the tissues of the embryo's body, as well as to the nontrophoblast tissues that support the developing embryo. The latter are referred to as extraembryonic tissues and include the yolk sac, allantois, and amnion. The trophectoderm, in turn, will generate the trophoblast cells of the chorion, the embryo's contribution to the extraembryonic tissue known as the placenta [19, 28].
The cells of the inner cell mass and trophectoderm continue to divide. Information gained from the study of mouse embryos suggests that the two tissues need to interact; the inner cell mass helps maintain the ability of trophectoderm cells to divide, and the trophectoderm appears to support the continued development of the inner cell mass [32]. Secreted paracrine factors (molecular signals that affect other cell types), including fibroblast growth factor-4 (FGF-4), which is released from inner cell mass cells [46], help direct embryogenesis at this stage. FGF-4 signaling also helps regulate the division and differentiation of trophectoderm cells [29].
By embryonic day 3 (E3.0) in the mouse and days 5 to 6 in human development [14], the embryo develops a cavity called the blastocoel. It fills with a watery fluid secreted by trophectodermal cells and transported in from the exterior. As a result of cavitation and the physical separation and differentiation of the trophectoderm from the inner cell mass, the morula becomes a blastocyst. Its chief structural features are the outer sphere of flattened trophectoderm cells (which become the trophoblast), the small, round cells of the inner cell mass, and the fluid-filled blastocoel [3, 19].
By E4.0 in mice, and between 5 to 7 days postfertilization in humans, the blastocyst reaches the uterus. It has not yet implanted into the uterine wall and is therefore still a pre-implantation embryo. When it arrives in the uterus, the blastocyst "hatches" out of the zona pellucida, the structure that originally surrounded the oocyte and that also prevented the implantation of the blastocyst into the wall of the oviduct [19]. (An embryo that does implant in the oviduct results in a tubal pregnancy, which can result in severe hemorrhaging.)
The nutritional requirements of the embryo change markedly during the time from zygote formation to the compaction of the morula, to the development of the blastocyst. Also, the physiology and biochemistry of the cells change as they increase in number and begin to differentiate. For example, the primary sources of energy for the cleavage-stage embryo are pyruvate, lactate, and amino acids—simple molecules that play important roles in various metabolic pathways. But after compaction of the morula, glucose is taken up by the embryo and used as a primary source of energy [15]. Indeed, mammalian blastocysts may have a unique transporter molecule, GLUT8, that ferries glucose into the blastocyst. GLUT8 appears in the blastocyst at the same time as the receptor for insulin-like growth factor–1 (IGF-1). Thus, the blastocyst, which requires a great deal of energy at this stage of development, is equipped to respond to insulin by taking up glucose [7].
These and other observations about the preimplantation blastocyst have led to recommendations about the importance of adapting the culture conditions to accommodate the changing nutritional requirements of the embryo when animal embryos are grown in the laboratory [16].
It is at this stage of embryogenesis—near the end of the first week of development in humans and about E4.0 in mice—that embryonic stem (ES) cells can be derived from the inner cell mass of the blastocyst. Human ES cells are derived from embryos generated through in vitro fertilization procedures and donated for research. An embryo at this stage of development in vivo would not yet be physically connected to the uterine wall; it would still be a preimplantation embryo.
ES cells, per se, may be an in vitro phenomenon. Some scientists argue that the apparent immortality of ES cells occurs only in a laboratory culture dish [41]. ES cells that are grown in the laboratory most closely resemble cells of the epiblast [5], but ES cells are not identical to epiblast cells [42]. The term epiblast refers to all the pluripotent cell populations that follow the formation of the primitive endoderm and precede the formation of the gastrula [23]. Like the epiblast cells of the embryo, ES cells in culture have the potential to give rise to all the cell types of the body. However, unlike the epiblast cells of the embryo, ES cells in vitro cannot give rise to a complete organism. They do not have the three-dimensional environment that is essential for embryonic development in vivo, and they lack the trophectoderm and other tissues that support fetal development in vivo (see Chapter 2. The Embryonic Stem Cell).
The Blastocyst Implants in the Uterine Wall
Many of the molecular and cellular events that occur during the second week of human embryonic development, and at the end of the first week of embryogenesis in the mouse, help establish the placenta. The placenta connects the fetal and maternal bloodstreams and provides nutrients to the embryo throughout the remainder of gestation.
On or about postfertilization days 8 to 9 in humans (and E4.5 in the mouse), the ball-shaped embryo implants into in the uterine wall (see Figure A.2. Development of the Preimplantation Blastocyst in Humans). The inner cell mass of the human embryo at this stage has split into layers. One is the hypoblast, which lies next to the blastocoel and gives rise to the primitive endoderm. (Later, the primitive endoderm will give rise to the outer layer of the yolk sac, a curious reminder of reptilian ancestry in mammalian embryos.) The other cell layer that develops from the inner cell mass is the epiblast. It will give rise to all the cells of the embryo's body [19, 23].
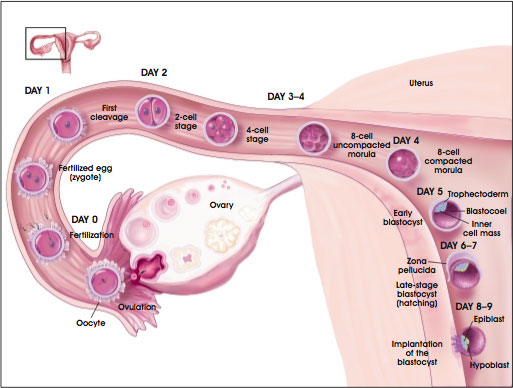
Figure A.2. Development of the Preimplantation Blastocyst in Humans.
(© 2001 Terese Winslow)
The epiblast can be thought of as the group of cells that succeeds the inner cell mass. Pluripotent cells are defined differently in scientific articles and text books. In general, however, pluripotent cells are capable of giving rise to all the kinds of cells that occur in the mature organism. So at this stage of embryogenesis, the only pluripotent cells are the undifferentiated cells of the epiblast.
By E6.0 in the mouse, three differentiated cell types exist: the trophoblast, the epiblast (also called the embryonic ectoderm or primitive ectoderm at this stage), and the primitive endoderm (see Figure A.3. Development of the Preimplantation Blastocyst in Mice). During the next major phase of development, termed gastrulation, the embryonic ectoderm will differentiate into the three primary germ layers—endoderm, mesoderm, and ectoderm. Thus, the embryonic ectoderm has succeeded the epiblast as the tissue that will generate the body of the embryo. The primitive endoderm differentiates into parietal and visceral endoderm, the anterior region of which will help regulate the development of the body plan during gastrulation [23].
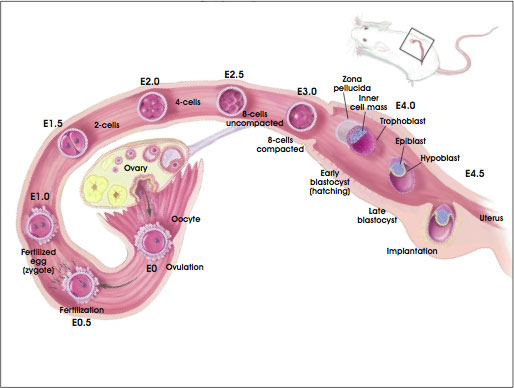
Figure A.3. Development of the Preimplantation Blastocyst in Mice from Embryonic Day 0 (E0) Through Day 5 (E5.0).
(© 2001 Terese Winslow)
Prior to gastrulation, the majority of cells (approximately 75 percent) in the preimplantation blastocyst comprise the trophectoderm and the primitive endoderm. The preimplantation mouse embryo consists of approximately 200 cells, approximately 20 to 25 of which are inner cell mass or epiblast cells [20, 23]. The day 5, preimplantation human embryo contains 200 to 250 cells, only 30 to 34 of which are inner cell mass cells [4].
The extraembryonic cells of both species differentiate into the tissues that will convey nutrients to the embryo and remove its waste products. For example, some of the trophoblast cells invade the epithelial lining of the uterus (also known as the decidua), and form a multinucleated tissue called a syncitium. This syncytiotrophoblast, as it is called, then develops lacunae (cavities). By postfertilization day 10 to11 in humans, the syncytiotrophoblast becomes supplied with maternal blood vessels. The fusion of the embryonic chorion and the maternal decidua and vascular tissue generates the placenta [19, 29].
The formation of the placenta is a critical process in human embryogenesis. Without a healthy placenta, the embryo does not survive; its malformation can trigger a spontaneous abortion [49]. The placenta anchors the developing embryo to the uterine wall and connects it to the maternal bloodstream, thus supplying the embryo with ions and metabolites and providing a waste-removal mechanism for the embryo [10, 26]. Later, the umbilical cord connects the embryo to the chorion portion of the placenta. The cord contains the fetal arteries and veins. Usually, the maternal and fetal blood do not mix directly. Instead, soluble substances pass through fingerlike projections called villi that have embedded in the uterine wall, and that have also developed from the trophoblast of the embryo [19].
In mice, some of the genes that regulate the development of the placenta have been identified. One is the Mash2 gene, which is expressed in the trophoblast cells of the embryo after it implants into the uterine wall. If Mash2 is inactivated, the placenta does not form and the embryo dies (at E10.5 in the mouse) [21, 45]. However, it is not known whether the same genes that regulate placenta formation in the mouse act in humans.
Meanwhile, during postfertilization days 7 to 14 of human development, the epiblast splits to form the amnionic cavity. The cavity fills with fluid and cushions the embryo throughout gestation.
The Blastocyst Becomes a Gastrula
At the start of the third week of human development, and about E6.0 in the mouse (the egg-cylinder stage), the cells of the epiblast begin to differentiate. By the end of the third week, they will have generated the three primary germ layers of the embryo—endoderm, mesoderm, and ectoderm. A detailed description of all the events of this critical stage of differentiation—known as gastrulation—is beyond the scope of this report. However, the onset of gastrulation is triggered at the posterior end of the embryo with the formation of a structure called the node (from Hensen's node in chick embryogenesis). The node, together with another important signaling center, the anterior visceral endoderm (AVE), helps regulate the formation of the pattern of the embryo's body at this stage of development [19].
The process of gastrulation begins between days 14 and 16 of human development and at about E6.5 in the mouse. At that time, a primitive streak forms in a specific region of the epiblast along the posterior axis of the embryo. Little is known about the signals that regulate the generation of the primitive streak, although the genes goosecoid, T, Evx-1, and follistatin are expressed [23]. Nevertheless, the forward migration of the posterior epiblast cells occurs as their cell-cell contacts break down, and they release enzymes that digest the basement membrane that lies underneath. This allows the epiblast cells to migrate into the space between the epiblast and the visceral endoderm [6].
The forward-moving epiblast cells also spread laterally, a migration that induces the formation of the mesoderm and the notochord. The notochord is a temporary, rod-like structure that develops along the dorsal surface of the embryo and will ultimately connect the anterior visceral endoderm (AVE) and the node. Cells at the anterior end of the notochord will eventually underlie the forebrain [19].
At the anterior end of the primitive streak is the node, a two-layered structure and important signaling center in the embryo. The ventral layer of cells in the node comes from the epiblast and generates the notochordal plate, which then forms the notochord. Endoderm, which will give rise to the gut, also develops near the node, along the sides of the notochord.
Meanwhile, the anterior region of the mesoderm that develops from the primitive streak is preparing to give rise to the heart. The anterior epiblast is generating the neuroectoderm and the ectoderm that covers the surface of the embryo. The ectodermal tissue that lies dorsal to the notochord will generate the neural plate, which will round up to form the neural tube, the precursor to the central nervous system (brain and spinal cord) [23].
Thus, by the end of the third week of embryonic development in humans, and by E8.0 in the mouse, the primitive ectoderm of the postimplantation blastocyst has generated the ectoderm, mesoderm, and endoderm of the gastrula (see Figure A.4. Development of Human Embryonic Tissues). These and other complex processes result in the formation of the tissues and organs that occur in an adult mammal (see Figure 1.1. Differentiation of Human Tissues). They require the activation and inactivation of specific genes at specific times, highly integrated cell-cell interactions, and interactions between cells and their noncellular environment, the extracellular matrix [3, 19].
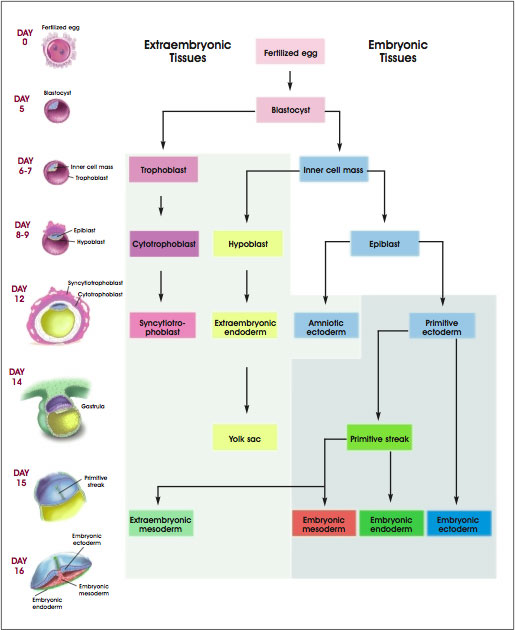
Figure A.4. Development of Human Embryonic Tissues.
(© 2001 Terese Winslow)
In general, the embryonic "outer" layer, or ectoderm, gives rise to the following tissues: central nervous system (brain and spinal cord) and peripheral nervous system; outer surface or skin of the organism; cornea and lens of the eye; epithelium that lines the mouth and nasal cavities and the anal canal; epithelium of the pineal gland, pituitary gland, and adrenal medulla; and cells of the neural crest (which gives rise to various facial structures, pigmented skin cells called melanocytes, and dorsal root ganglia, clusters of nerve cells along the spinal cord). The embryonic "middle" layer, or mesoderm, gives rise to skeletal, smooth, and cardiac muscle; structures of the urogenital system (kidneys, ureters, gonads, and reproductive ducts); bone marrow and blood; fat; bone, and cartilage; other connective tissues; and the lining of the body cavity. The embryonic "inner" layer, or endoderm, gives rise to the epithelium of the entire digestive tract (excluding the mouth and anal canal); epithelium of the respiratory tract; structures associated with the digestive tract (liver and pancreas); thyroid, parathyroid, and thymus glands; epithelium of the reproductive ducts and glands; epithelium of the urethra and bladder [19].
Primordial Germ Cells Are the Precursors to Eggs and Sperm
Not to be forgotten in this developmental scheme are the primordial germ (PG) cells, which will give rise to eggs and sperm in the adult organism (see Figure A.5. Development of Mouse Embryonic Primordial Germ Cells). Prior to gastrulation, at about the time of primitive streak formation, these precursor cells split off from the proximal region of the epiblast and migrate into the extraembryonic mesoderm (which generates the yolk sac and allantois). It is not until the proximal epiblast cells reach the extraembryonic mesoderm that they are committed to becoming PG cells. Their location in this tissue—which is remote from the rest of the embryo's body, or somatic, cells—may allow PG cells to avoid some of the events that drive somatic cells through the process of differentiation. One such event is DNA methylation, a means of silencing genes inherited from one parent—a process termed genomic imprinting (discussed below).
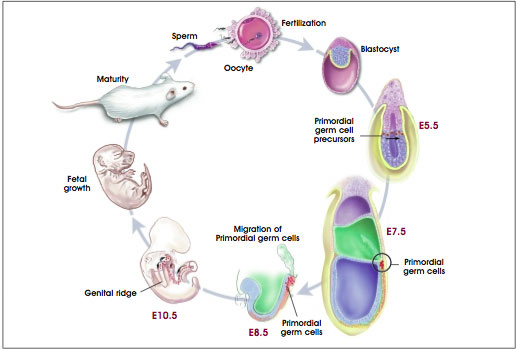
Figure A.5. Development of Mouse Embryonic Primordial Germ Cells.
(© 2001 Terese Winslow, Caitlin Duckwall)
Another feature that distinguishes primordial germ cells from somatic cells is their continuous expression of Oct-4, a transcription factor produced by proliferating, unspecialized cells. Thus, the regulation of PG cell fate in the mammalian embryo is a result of the local environment of the cells, a recurring theme in mammalian embryogenesis, and the expression of genes in the PG cells [37]. Later in development, the PG cells embark in another migration and ultimately come to rest in the genital ridge, the tissue that will give rise to the gonads: testes in males and ovaries in females [35]. In the testis, the PG cells give rise to spermatagonial stem cells that reside in the testis throughout the life of the male. They continuously renew themselves and differentiate through the process of spermatogenesis into mature, functional sperm cells. There is no evidence, however, that they have pluripotential properties [39].
Genes, Molecules, and Other Signals Are Important in Early Embryogenesis
This overview of the processes of blastocyst formation, implantation, and gastrulation has ignored most of the crucial signals that direct embryonic development. These signals include genes expressed by cells at different stages of development, molecular factors secreted by cells, complex molecular signaling systems that allow cells to respond to secreted factors, specialized membrane junctions that connect cells and allow them to communicate, components of the noncellular environment (known as the extracellular matrix), and genomic imprinting. These signals—as well as their origins and effects—are the least understood elements of embryonic development in any organism.
Gene Transcription, Translation, and Protein Synthesis
A gene is a linear segment of a DNA molecule that encodes one or more proteins. The process occurs in three major steps (see Figure A.6. Gene Transcription, Translation, and Protein Synthesis). The DNA, which is double-stranded, unwinds and copies its triplet code (varying sequences of the four nitrogen bases adenine (A), thymine (T), cytosine (C), and guanine (G)) into a messenger RNA (mRNA) molecule. In RNA, uracil (U) is substituted for T. The process is called transcription because the triplet code of a DNA molecule is transcribed into the triplet code of an mRNA molecule. A gene that makes an mRNA transcript is active; the gene is said to be expressed.
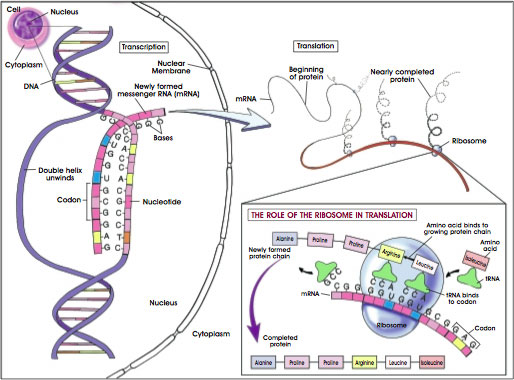
Figure A.6. Gene Transcription, Translation, and Protein Synthesis.
(© 2001 Terese Winslow)
The process of initiating transcription is complex. It requires the binding of certain proteins, called transcription factors, to regions of the DNA near the site where transcription begins. Transcription factors bind at sequences of DNA called the promoter enhancer region. The factors can activate or repress transcription. Although some transcription factors bind directly to the DNA molecule, many bind to other transcription factors. Thus, protein-DNA interactions and protein-protein interactions regulate gene activity. Their interactions then activate or block the process of transcription.
Transcription actually begins when the enzyme RNA polymerase II binds to the promoter region of DNA to initiate the process of making a molecule of messenger mRNA. As indicated above, the sequence of bases in DNA—the order of A, T, C, and G—dictates the sequence of the mRNA which will be formed. Thus, an A in DNA can bind only to a U in mRNA. The DNA base G will bind only to the RNA base C, and so on. RNA polymerase connects these bases together in a process called elongation.
The second major stage of the process of making proteins based on the code of DNA is called translation. During translation, the mRNA—which was generated in the nucleus of a cell and now carries its transcript of the DNA code—moves to the cytoplasm, where it attaches temporarily to tiny structures called ribosomes. There, molecules of mRNA direct the assembly of small molecules called amino acids (of which 20 kinds exist) into proteins. Each amino acid is specified by a code of three bases. The helpers in this effort are molecules of transfer RNA (tRNA). Each tRNA molecule contains its own triplet code (to match the mRNA code), and each tRNA ferries a particular kind of amino acid to the mRNA-laden ribosomes.
Then, in the third step of protein synthesis, the amino acids are linked through chemical bonds to create a protein molecule. Proteins typically consist of hundreds of amino acids. Thus, the sequence of bases in DNA determines the sequence of mRNA, which then determines the linear sequence of amino acids in a protein. Depending on its sequence of amino acids, a protein may fold, twist, bend, pleat, coil, or otherwise contort itself until it assumes the three-dimensional shape that makes it functional.
In the body, proteins make up most of the structural elements of cells and tissues. They also function as enzymes, which regulate all of the body's chemical reactions.
Gene Expression and Factors in the Preimplantation Blastocyst
It is difficult to identify the genes and factors in vivo that affect the earliest events in mammalian development; maintain the undifferentiated, proliferating state of inner cell mass or epiblast cells; regulate implantation; and direct the differentiation of cells along specific developmental pathways, or cell lineages. The embryo itself is very small and, in vivo, is almost wholly inaccessible to study. Therefore, many of the genetic and molecular influences that are now known to regulate early embryogenesis in vivo were identified by studying mouse embryonic stem cells in vitro.
For instance, Oct-4 is a transcription factor that has come to be recognized as a prototypical marker of undifferentiated, dividing cells. It is necessary for maintaining the undifferentiated state and proliferation of cells of the inner cell mass and the epiblast. Most of the studies of Oct-4 have been conducted in mouse embryos and ES cells. Oct-4 is expressed in the mouse oocyte, it disappears during the first cleavage of the zygote, and it reappears in the four-cell mouse embryo as the genome of the zygote begins to control embryonic development. Oct-4 persists in the inner cell mass of the blastocyst, but does not occur in differentiated trophectoderm cells, nor does it occur other differentiated cell types that arise after gastrulation in the mouse. The gene for Oct-4, Pou5f1, is expressed in primordial germ cells, however [12, 32].
Oct-4 is a member of the class 5 POU (for Pit, Oct, and Unc) family of transcription factors, which bind promoter or enhancer sites in DNA. These proteins regulate gene transcription. The transcription factor Oct-4 can activate or repress gene expression; it binds to DNA at a distance from the start of transcription. Hence, depending on the target gene, Oct-4 may require the presence of co-activator proteins such as the E1A-like transcription factors and the Sox2 protein [37].
Another target of Oct-4 in mouse embryogenesis is the Fgf4 gene. It encodes fibroblast growth factor-4 (FGF-4), a growth factor protein that is expressed together with Oct-4 in the inner cell mass and epiblast [32]. FGF-4 is a paracrine signal, meaning that it is released from one cell type and it acts on another. In this case, FGF-4 is released from proliferating inner cell mass cells and it affects the surrounding trophectoderm. FGF-4 may also act as an autocrine signal, meaning that it may affect the same inner cell mass cells that released it [13].
A series of recent experiments indicates that the level of Oct-4 expression—not simply its presence or absence in a cell—determines how mouse embryonic stem cells differentiate and whether they continue to proliferate [33] (see Appendix B. Mouse Embryonic Stem Cells).
Two proteins, leptin and STAT3, which are produced by maternal granulosa cells that surround the oocyte, are apparently secreted into the oocyte as it matures in its ovarian follicle. By the time the mouse or human zygote reaches the four-cell stage, leptin and STAT3 are concentrated in what may be the founder cell of the embryonic trophectoderm. Later, when the trophectoderm differentiates and separates from the inner cells mass of the blastocyst, leptin and STAT3 are expressed only in the trophectoderm, where they play a critical role during implantation [12].
Leukemia inhibitory factor (LIF), a cytokine, also plays an important role during implantation. The LIF gene is expressed in cultured mouse [31] and bovine blastocysts, as is the gene for its receptor. The mRNA for the receptor for LIF is expressed in human blastocysts [12, 47]. LIF, therefore, seems to be important for early mammalian blastocyst development, as well as implantation. It is also essential for the survival of the primordial germ cells, which will become eggs and sperm in the mature organism [22]. And if mouse embryonic stem (ES) cells are cultured from the inner cell mass of a blastocyst without the presence of "feeder" layers of cells, they require the addition of LIF to the culture medium in order to survive and proliferate [40]. Curiously, cultures of human ES cells do not respond to LIF [36, 48].
Regulation of Body Patterning in the Embryo
As the embryo forms, its overall body pattern is determined by the establishment of three clear axes—the anterior-posterior axis (head-tail), the dorsal-ventral (back-belly) axis, and left-right asymmetry. The establishment of these body axes at the correct time is fundamental to normal embryonic development. For instance, the central nervous system develops along the dorsal surface, with the largest concentration of neuronal tissue—the brain—at the anterior end of the embryo. The limbs develop symmetrically and bilaterally, whereas the heart—although it begins as a symmetrical structure—ultimately comes to point toward the left side of the trunk. Some internal structures are paired (the kidneys, lungs, adrenal glands, testes, and ovaries), whereas many are not (the heart, gut, pancreas, spleen, liver, and uterus) [19].
Information about the establishment of these body axes and their role in development is far from complete. For example, the anterior-posterior axis of the mouse blastocyst may be determined before it implants and is certainly established before gastrulation [15, 17]. An unanswered question, however, is whether this early embryonic axis helps determine the later development of the embryo. The early axis may play a role in primitive streak formation, and requires the expression of Wnt, which helps regulate the formation of one of embryo's chief signaling centers: the node [38].
As indicated above, an important group of cells that produces molecular signals that help determine the anterior-posterior axis of the mouse embryo is the anterior visceral endoderm (AVE). The AVE expresses different genes along its length. At E5.0 in the mouse, for example, the Hex gene—a member of the family of homeobox genes that help regulate body patterning of the mouse embryo—is expressed in the distal visceral endoderm. These cells migrate to become the AVE, which forms on the opposite side of the embryo from the primitive streak, thus establishing the anterior-posterior axis of the fetus [17].
Then, between E6.0 and E7.0 in the mouse, the anterior region of the AVE, where the heart will form, expresses Mrg1. The medial region expresses the transcription factor genes Otx2 and Lim1, as well as other genes. The region of the AVE that lies next to the part of the epiblast that will give rise to oral ectoderm and the forebrain expresses Hesx1, another homeobox gene. Collectively, the AVE and the genes it expresses help regulate the development of the anterior end of the embryo [3].
Other genes, notably Bmp4, also help shape the mouse embryo prior to gastrulation. BMP stands for bone morphogenetic protein, a family of proteins that help regulate the differentiation of mesenchymal cells, which are derived from mesoderm, including bone-forming osteoblasts, and adipocytes, which are fat cells. They also play a role in CNS development. Bmp4, which is expressed in the extraembryonic ectoderm next to the epiblast and also in the inner cell mass of the E3.5 and E4.5 mouse blastocyst, may activate genes in epiblast cells that then migrate to form the primitive streak. Wnt3 apparently helps induce the formation of both the primitive streak and the node in mammals, although there is no evidence indicating that Wnt3 expression is required for mesoderm induction. However, formation of the embryo's head region, obviously a key anterior structure, seems to require inhibition of the activities of Wnt and Bmp4—a potential role of the AVE [3, 18].
Therefore, coordinating the embryo's "decisions" about its body pattern is a hierarchy of genes. Overall, the Hox genes specify anterior-posterior polarity. Their normal function can be subverted by retinoic acid, which can activate Hox genes in inappropriate places. Less is known about the establishment of the dorsal-ventral axis. It may be determined in the blastocyst, or even in the oocyte [16]; it is clearly established when the notochord develops. Genes such as Nodal and Lefty help determine left-right asymmetry. Genes that regulate body patterning in embryonic development are well conserved throughout evolution among both vertebrates and invertebrates [19].
Regulation of Cell Differentiation in Early Embryogenesis
Myriad other genetic and molecular signals conspire to regulate cell differentiation in the embryo. Factors in a cell's environment bind to receptor molecules in its membrane and activate a series of intracellular responses that may result in gene activation or inactivation. The process by which a cell responds to an external signal is called signal transduction, and is itself the subject of many articles and books.
One of the earliest genes to be involved in cell differentiation in the preimplantation blastocyst are those that encode the GATA class of transcription factors. GATA-6 is expressed in some inner cell mass cells of the E3.5 mouse blastocyst; GATA-4 is expressed in the E5.5 parietal and visceral endoderm. GATA-6 expression is required for the formation of the visceral endoderm; the role of GATA-4 is less clear. Other genes such as HNF-4, which encodes a transcription factor, and STAT3, which encodes a protein important in a cytokine signaling pathway, are expressed later, during the early differentiation of the visceral endoderm [18].
Other genes are expressed in the pregastrulation epiblast; examples are Brachyury and Cripto, which encode secreted growth factors. Still others, including Nodal and Otx2, are expressed in both the epiblast and the visceral endoderm [18].
A host of genes is expressed along the primitive streak. These include HNF-3β in the notochord, node, and floor plate (which will underlie the forebrain); nodal, goosecoid, T, and Lim-1, in the node; Follistatin and T for the remainder of the streak; and FGF-4 just caudal to the node.
It is far easier to monitor the expression of particular genes than it is to identify their function(s) during development. One of the most useful kinds of experiments for determining the function of a gene involves its permanent inactivation—to create a knockout mouse, for example—followed by studies of impaired functions in the gene-deficient animal. Similar research strategies obviously cannot be used to determine the functions of specific genes in human embryogenesis. However, it is possible to identify human genes that are important for development by studying heritable abnormalities or congenital defects that have a genetic basis. Then, the function of the human genes—which almost certainly will have similar effects in mice—can be assessed in more detail by generating knockout mice that lack the gene.
The Cell Cycle
Many cells of the early embryo are in a constant state of dividing or of preparing to divide. The series of molecular events that regulate these processes is called the cell cycle (see Figure A.1. Cell Cycle).
The cell cycle includes four main phases: DNA synthesis (S phase), G2 (a gap phase during which the cell increases in size and prepares to divide), cell division (also called mitosis, M phase), and G1 (a gap phase of cell growth and replication of the centrioles). When a cell exits the cell cycle, to differentiate, for example, it is said to be in G0. Progression through the cell cycle is regulated by the activation of cyclindependent kinases (Cdks), enzymes that attach phosphate groups onto other proteins. Particular Cdks and their associated cyclins regulate the transition from one phase of the cell cycle to the next. For example, in mammalian cells, Cdk2 and cyclin E regulate the transition from G1 to S, whereas Cdk1 and cyclins A and B regulate the transition from S to G2. And recently, it has become clear that the cell cycle has several checkpoint mechanisms, during which the cell stops its progression through the cycle while it repairs damaged DNA [11].
The activity of the cell cycle varies, depending on the status of the cell and the cues—such as cytokine stimulation—the cell receives from its environment. Some cells "cycle" quickly, dividing in a matter of hours. Others cycle slowly, and some do not cycle at all. The epiblast cells of the postimplantation E5.5 to E6.0 mouse blastocyst, for example, have a mean cycle time of 11.5 hours. But a day later, at E6.5 to E7.0, epiblast cells have a mean cell cycle time of only 4.4 hours [23]. In contrast, the cycle time for cells in the cleavage-stage human embryo—a much earlier developmental stage—is approximately 36 hours [34]. And cells that are terminally differentiated—mature nerve cells in the brain, for example—have stopped dividing altogether. What factors regulate the cell cycle during development, or how the cell cycle alters gene expression or any other event in embryogenesis, remains largely unknown.
Cell Death Is a Normal Process During Embryogenesis
It is a general characteristic of undifferentiated cells—including embryonic cells in vivo or in vitro—that when they stop dividing, they differentiate, become quiescent or senescent (stop their progress through the cell cycle and enter a period of temporary or permanent "rest"), or die. In vivo or in vitro, the process of cell death can occur by necrosis or apoptosis. The latter is a form of genetically controlled cell death that, in itself, is an important aspect of normal embryonic development in vivo. When the genetic program for apoptosis becomes activated, the cell commits a form of molecular suicide. Its DNA disintegrates in a characteristic manner, blebs (small pouches) form in the cell membrane, and the cell dies. The genetic controls for apoptosis differ, depending on the cell type, but all involve activating proteases called caspases, enzymes that destroy the protein components of cells.
As the body of an embryo develops, apoptosis helps shape it. For example, apoptosis helps control the spacing of nerve cells in the brain and spinal cord; it helps generate the space in the middle ear, and it causes the death of skin cells between fingers and toes—the typical "webbing" of fetal digits [19].
Many of the genes that regulate apoptosis were discovered in studies of the microscopic roundworm, C. elegans. The mammalian counterparts of these genes are very similar in terms of their DNA sequences, and are called homologues. For example, in C. elegans, the ced-4 and ced-3 genes are activated (in that order) prior to apoptosis. They, in turn, activate enzymes called caspases, which actually trigger apoptosis. But the regulatory pathway that leads to cell death is complex. Another apoptosis-control gene called ced-9 can block activation of ced-4 and ced-3, and thereby "rescue" a cell from apoptosis. The mammalian homologues of ced-9 are members of the BCL-2 gene family, which prevent apoptosis in mammalian cells—and in C. elegans, if they are introduced into cells from the worm [19].
Another mammalian apoptosis gene, Apaf-1 works with caspase-9 to bring about cell death. It is interesting to note that the silencing of the Apaf-1 gene—rather than a mutation in its DNA sequence—was recently linked to cancer metastasis [43]. In fact, several of the genes that normally regulate apoptosis inhibit the formation of tumors because they trigger the death of cells with damaged DNA that might otherwise replicate to produce a tumor. Because of their normal, protective function against the development of cancer, such genes are termed tumor-suppressor genes. Many tumor-suppressor genes, including Apaf-1, are associated with the so-called p53 tumor-suppressor pathway. If even one of the apoptosis-regulating genes becomes mutated, the tumor-suppressor pathway can fail, a step toward the development of cancer.
Some Comparisons Between Embryogenesis and Oncogenesis
There are many molecular links between the regulation of normal embryogenesis and the induction of cancer, which is called oncogenesis. A comprehensive review of the similarities between the two exceeds the scope of this report. However, it is useful to point out that at least some of the genes, factors, and cell-cell interactions critical for normal embryonic development also play a role in—or are altered in—tumor development. The example cited above indicates that some of the genes that function during apoptosis in the embryo also protect the mature organism from developing tumors.
A different, and obvious, parallel between embryogenesis and oncogenesis can be observed in the spontaneous formation of tumors in the gonads of mammals, including humans. These unusual tumors, which include teratomas, embryonal carcinomas, and teratocarcinomas, develop from the germ cells in the testes or ovaries. The tumors have provoked a great deal of interest because they often contain highly differentiated cells and tissues such as teeth, hair, neural cells, and epithelial cells. The structures are disorganized, but often recognizable [2].
Although teratomas are benign, embryonal carcinomas and teratocarcinomas are highly malignant. The latter contain a kind of stem cell, called an embryonal carcinoma (EC) cell, which in mice and humans resembles embryonic stem (ES) cells. Human EC cells, unlike ES cells, typically have abnormal chromosomes. The chromosomes in mouse EC cells may appear to be normal, although they carry genetic defects. Nevertheless, mouse EC cells can contribute to normal embryonic development if they are introduced into a mouse blastocyst, which is then implanted in the uterus of a pseudopregnant female [2] (see Table A.1. Comparison of Mouse, Monkey, and Human ES, EG, and EC Cells).
Other genes recently identified as important in the development of human cancers are also active during embryonic development. For instance, the human breast cancer genes, BRCA1 and BRCA2, and their counterparts in mice are expressed in the three primary germ layers during embryogenesis, particularly in cell types undergoing the most rapid proliferation. The expression of these genes is dependent on the stage of the cell cycle, with peak expression during the G1/S transition and lowest expression in cells in the G1 or G0 phase. In mouse and nonhuman primate (cynomolgus monkey) embryos, the temporal and spatial patterns of Brca1 and Brca2 expression are virtually identical, despite the fact that the coding sequences for the genes and their promoters differ between the species. In humans, BRCA1 and BRCA2 probably function during the development of mammary epithelium, although little is known about their role in this process. Mutant forms of the genes appear to cause breast cancer only if the mutations occur in the germ line. Somatic mutations of BRCA1 and BRCA2 are not linked to breast cancer [8].
DNA Methylation and Genomic Imprinting Affect Embryonic Development
DNA methylation is the process of adding methyl groups to specific cytosine residues in the promoter regions of DNA. DNA methylation is a genome-wide phenomenon; it occurs in many genes depending on the stage of development and the differentiation status of a cell. When the methyl groups are bound at their designated sites in DNA, transcription factors cannot bind to the DNA and gene transcription is turned off. Also, DNA methylation causes a rearrangement of the structure of chromatin, the combination of DNA and protein that forms the chromosomes. DNA methylation patterns change during development, and their rearrangement in different tissues at different times is an important method for controlling gene expression [19].
Also important to embryonic development is the process of genomic imprinting, which causes certain to be genes turned on or off, depending on whether they are inherited from the mother or the father. Several mechanisms of genomic imprinting exist in mammals. A common method of imprinting is DNA methylation. Once methylated, or "marked," a gene may be activated or inactivated. Thus, the process of marking a gene as being inherited from either the father or the mother is genomic imprinting [19].
For most of the genes known to undergo imprinting, specific regulatory regions have been identified where methylation takes place. The methylation marks are acquired during gametogenesis, the process of sperm and egg formation, and they persist during the development of the pre- and post-implantation embryo [9, 44]. In contrast, the genes of nonimprinted embryos acquire their methylation patterns after blastocyst implantation, as do ES cells in vitro [50].
The genomes of germ cells and the zygote are largely demethylated, although the sites associated with parental-specific imprints remain methylated. In the preimplantation blastocyst, the nonimprinted genes of undifferentiated cells remain demethylated, which means that most of their genes are capable of being expressed. But before gastrulation, as the three germ layers prepare to differentiate, the DNA of the embryo's somatic cells becomes remethylated and genes are selectively turned on or off. The only cells that escape this phenomenon are the primordial germ cells (PGCs). They gradually remove their genomic imprinting marks, which exist in the form of parentally specified DNA methylation patterns. This phenomenon of erasing the marks for genomic imprinting occurs as the PGCs migrate to the gonadal ridges, which in the mouse occurs on E13.5 [23]. Then, as the germ cells mature, their genomes acquire new imprints due to the activity of a specific DNA methyltransferase, which adds methyl groups to DNA [50].
Table A.1. Comparison of Mouse, Monkey, and Human Pluripotent Stem Cells
Marker
Name |
Mouse EC/
ES/EG cells |
Monkey
ES cells |
Human
ES cells |
Human
EG cells |
Human
EC cells |
| SSEA-1 |
+ |
– |
– |
+ |
– |
| SSEA-3 |
– |
+ |
+ |
+ |
+ |
| SEA-4 |
– |
+ |
+ |
+ |
+ |
| TRA-1–60 |
– |
+ |
+ |
+ |
+ |
| TRA-1–81 |
– |
+ |
+ |
+ |
+ |
| Alkaline phosphatase |
+ |
+ |
+ |
+ |
+ |
| Oct-4 |
+ |
+ |
+ |
Unknown |
+ |
| Telomerase activity |
+ ES, EC |
Unknown |
+ |
Unknown |
+ |
| Feeder-cell dependent |
ES, EG, some EC |
Yes |
Yes |
Yes |
Some; relatively low clonal efficiency |
| Factors which aid in stem cell self-renewal |
LIF and other factors that act through gp130 receptor and can substitute for feeder layer |
Co-culture with feeder cells; other promoting factors have not been identified |
Feeder cells + serum; feeder layer + serum-free medium + bFGF |
LIF, bFGF, forskolin |
Unknown; low proliferative capacity |
| Growth characteristics in vitro |
Form tight, rounded, multi-layer clumps; can form EBs |
Form flat, loose aggregates; can form EBs |
Form flat, loose aggregates; can form EBs |
Form rounded, multi-layer clumps; can form EBs |
Form flat, loose aggregates; can form EBs |
| Teratoma formation in vivo |
+ |
+ |
+ |
– |
+ |
| Chimera formation |
+ |
Unknown |
+ |
– |
+ |
KEY
ES cell = Embryonic stem cell
EG cell = Embryonic germ cell
EC cell = Embryonal carcinoma cell
SSEA = Stage-specific embryonic antigen |
TRA = Tumor rejection antigen-1
LIF = Leukemia inhibitory factor
bFGF = Basic fibroblast growth factor
EB = Embryoid bodies |
References
- Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K., and Watson, J.D. (1994). Molecular biology of the cell, (New York: Garland Publishing, Inc.).
- Andrews, P.W., Przyborski, S.A., and Thomson, J. (2001). Embryonal carcinoma cells as embryonic stem cells. Marshak, D.R., Gardner, D.K., and Gottlieb, D. eds. Cold Spring Harbor Laboratory Press, 231–266.
- Beddington, R.S. and Robertson, E.J. (1999). Axis development and early asymmetry in mammals. Cell. 96, 195–209.
- Bongso, A., personal communication.
- Brook, F.A. and Gardner, R.L. (1997). The origin and efficient derivation of embryonic stem cells in the mouse. Proc. Natl. Acad. Sci. U. S. A. 94, 5709–5712.
- Burdsal, C.A., Flannery, M.L., and Pedersen, R.A. (1998). FGF-2 alters the fate of mouse epiblast from ectoderm to mesoderm in vitro. Dev. Biol. 198, 231–244.
- Carayannopoulos, M.O., Chi, M.M., Cui, Y., Pingsterhaus, J.M., McKnight, R.A., Mueckler, M., Devaskar, S.U., and Moley, K.H. (2000). GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc. Natl. Acad. Sci. U. S. A. 97, 7313–7318.
- Chodosh, L.A. (1998). Expression of BRCA1 and BRCA2 in normal and neoplastic cells. J. Mammary Gland Biol. Neoplasia. 3, 389–402.
- Constância, M., Pickard, B., Kelsey, G., and Reik, W. (1998). Imprinting mechanisms. Genome Res. 8, 881–900.
- Cronier, L., Bastide, B., Defamie, N., Niger, C., Pointis, G., Gasc, J.M., and Malassine, A. (2001). Involvement of gap junctional communication and connexin expression in trophoblast differentiation of the human placenta. Histol. Histopathol. 16, 285–295.
- D'Urso, G. and Datta, S. (2001). Cell cycle control, checkpoints, and stem cell biology. Marshak, D.R., Gardner, D.K., and Gottlieb, D. eds. Cold Spring Harbor Laboratory Press, 61–94.
- Edwards, R.G. (2000). The role of embryonic polarities in preimplantation growth and implantation of mammalian embryos. Hum. Reprod. 15 Suppl 6, 1–8.
- Feldman, B., Poueymirou, W., Papaioannou, V.E., DeChiara, T.M., and Goldfarb, M. (1995). Requirement of FGF-4 for postimplantation mouse development. Science. 267, 246–249.
- Fong, C.Y., Bongso, A., Ng, S.C., Kumar, J., Trounson, A., and Ratnam, S. (1998). Blastocyst transfer after enzymatic treatment of the zona pellucida: improving in-vitro fertilization and understanding implantation. Hum. Reprod. 13, 2926–2932.
- Gardner, R.L. (1997). The early blastocyst is bilaterally symmetrical and its axis of symmetry is aligned with the animal-vegetal axis of the zygote in the mouse. Development. 124, 289–301.
- Gardner, D.K. (1998). Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology. 49, 83–102.
- Gardner, R.L. (1999). Polarity in early mammalian development. Curr. Opin. Genet. Dev. 9, 417–421.
- Gardner, R.L. (2001). The initial phase of embryonic patterning in mammals. Int. Rev. Cytol. 203, 233–290.
- Gilbert, S.F. (2000). Developmental biology. (Sunderland, MA: Sinauer Associates).
- Gossler, A. (1992). Early embryonic development of animals, Hennig, W., Nover, L., and Scheer, U. eds. (Berlin, New York: Springer-Verlag).
- Guillemot, F., Nagy, A., Auerbach, A., Rossant, J., and Joyner, A.L. (1994). Essential role of Mash-2 in extraembryonic development. Nature. 371, 333–336.
- Hara, T., Tamura, K., de Miguel, M.P., Mukouyama, Y., Kim, H., Kogo, H., Donovan, P.J., and Miyajima, A. (1998). Distinct roles of oncostatin M and leukemia inhibitory factor in the development of primordial germ cells and sertoli cells in mice. Dev. Biol. 201, 144–153.
- Hogan, B., Beddington, R., Constantini, F., and Lacy, E. (1994). Manipulating the mouse embryo a laboratory manual, (Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press).
- Hogan, B.L. (1999). Morphogenesis. Cell. 96, 225–233.
- Hogan, B. (2001). Primordial germ cells as stem cells. Marshak, D.R., Gardner, D.K., and Gottlieb, D. eds. Cold Spring Harbor Laboratory Press, 189–204.
- Janatpour, M.J., Utset, M.F., Cross, J.C., Rossant, J., Dong, J., Israel, M.A., and Fisher, S.J. (1999). A repertoire of differentially expressed transcription factors that offers insight into mechanisms of human cytotrophoblast differentiation. Dev. Genet. 25, 146–157.
- Johnson, M.H., Maro, B., and Takeichi, M. (1986). The role of cell adhesion in the synchronization and orientation of polarization in 8-cell mouse blastomeres. J. Embryol. Exp. Morphol. 93, 239–255.
- Jones, J.M. and Thomson, J.A. (2000). Human embryonic stem cell technology. Semin. Reprod. Med. 18, 219–223.
- Kunath, T., Strumpf, D., Rossant, J., and Tanaka, S. (2001). Trophoblast stem cells. Marshak, D.R., Gardner, D.K., and Gottlieb, D. eds. Cold Spring Harbor Laboratory Press, 267–288.
- Marshak, D.R., Gottlieb, D., Kiger, A.A., Fuller, M.T., Kunath, T., Hogan, B., Gardner, R.L., Smith, A., Klar, A.J.S., Henrique, D., D'Urso, G., Datta, S., Holliday, R., Astle, C.M., Chen, J., Harrison, D.E., Xie, T., Spradling, A., Andrews, P.W., Przyborski, S.A., Thomson, J.A., Kunath, T., Strumpf, D., Rossant, J., Tanaka, S., Orkin, S.H., Melchers, F., Rolink, A., Keller, G., Pittenger, M.F., Marshak, D.R., Flake, A.W., Panicker, M.M., Rao, M., Watt, F.M., Grompe, M., Finegold, M.J., Kritzik, M.R., Sarvetnick, N., and Winton, D.J. (2001e). Stem cell biology, Marshak, D.R., Gardner, R.L., and Gottlieb, D. eds. (Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press).
- Nichols, J., Davidson, D., Taga, T., Yoshida, K., Chambers, I., and Smith, A. (1996). Complementary tissue-specific expression of LIF and LIF-receptor mRNAs in early mouse embryogenesis. Mech. Dev. 57, 123–131.
- Nichols, J., Zevnik, B., Anastassiadis, K., Niwa, H., Klewe-Nebenius, D., Chambers, I., Scholer, H., and Smith, A. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 95, 379–391.
- Niwa, H., Miyazaki, J., and Smith, A.G. (2000). Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 24, 372–376.
- Odorico, J.S., Kaufman, D.S., and Thomson, J.A. (2001). Multilineage Differentiation from Human Embryonic Stem Cell Lines. Stem Cells. 19, 193–204.
- Pelton, T.A., Bettess, M.D., Lake, J., Rathjen, J., and Rathjen, P.D. (1998). Developmental complexity of early mammalianpluripotent cell populations in vivo and in vitro. Reprod. Fertil. Dev. 10, 535–549.
- Pera, M.F., Reubinoff, B., and Trounson, A. (2000). Human embryonic stem cells. J. Cell Sci. 113 ( Pt 1), 5–10.
- Pesce, M., Anastassiadis, K., and Scholer, H.R. (1999). Oct-4: lessons of totipotency from embryonic stem cells. Cells Tissues Organs. 165, 144–152.
- Rossant, J., personal communication.
- Shinohara, T., Orwig, K.E., Avarbock, M.R., and Brinster, R.L. (2000). Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc. Natl. Acad. Sci. U. S. A. 97, 8346–8351.
- Smith, A.G., Heath, J.K., Donaldson, D.D., Wong, G.G., Moreau, J., Stahl, M., and Rogers, D. (1988). Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 336, 688–690.
- Smith, A. (2001). Embryonic stem cells. Marshak, D.R., Gardner, D.K., and Gottlieb, D. eds. Cold Spring Harbor Laboratory Press, 205–230.
- Smith, A.G. (2001). Origins and properties of mouse embryonic stem cells. Annu. Rev. Cell. Dev. Biol. 1–22.
- Soengas, M.S., Capodieci, P., Polsky, D., Mora, J., Esteller, M., Opitz-Araya, X., McCombie, R., Herman, J.G., Gerald, W.L., Lazebnik, Y.A., Cordon-Cardo, C., and Lowe, S.W. (2001). Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 409, 207–211.
- Surani, M.A. (1998). Imprinting and the initiation of gene silencing in the germ line. Cell. 93, 309–312.
- Tanaka, M., Gertsenstein, M., Rossant, J., and Nagy, A. (1997). Mash2 acts cell autonomously in mouse spongiotrophoblast development. Dev. Biol. 190, 55–65.
- Tanaka, S., Kunath, T., Hadjantonakis, A.K., Nagy, A., and Rossant, J. (1998). Promotion of trophoblast stem cell proliferation by FGF4. Science. 282, 2072–2075.
- Teruel, M., Smith, R., and Catalano, R. (2000). Growth factors and embryo development. Biocell. 24, 107–122.
- Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S., and Jones, J.M. (1998). Embryonic stem cell lines derived from human blastocysts. Science. 282, 1145–1147.
- Trounson, A.O., Gardner, D.K., Baker, G., Barnes, F.L., Bongso, A., Bourne, H., Calderon, I., Cohen, J., Dawson, K., Eldar-Geve, T., Gardner, D.K., Graves, G., Healy, D., Lane, M., Leese, H.J., Leeton, J., Levron, J., Liu, D.Y., MacLachlan, V., Munné, S., Oranratnachai, A., Rogers, P., Rombauts, L., Sakkas, D., Sathananthan, A.H., Schimmel, T., Shaw, J., Trounson, A.O., Van Steirteghem, A., Willadsen, S., and Wood, C. (2000). Handbook of in vitro fertilization, (Boca Raton, London, New York, Washington, D.C.: CRC Press).
- Tucker, K.L., Beard, C., Dausmann, J., Jackson-Grusby, L., Laird, P.W., Lei, H., Li, E., and Jaenisch, R. (1996). Germ-line passage is required for establishment of methylation and expression patterns of imprinted but not of nonimprinted genes. Genes Dev. 10, 1008–1020.
 Chapter 11 | Table of Contents | Appendix B
Chapter 11 | Table of Contents | Appendix B 
Historical content: June 17, 2001