Cocaine and the Changing Brain
Cocaine Causes Long-Term Synaptic Modulation In The Ventral Tegmental Area
John T. Williams, Ph.D.
Oregon Health Sciences University
Portland, OR
Acute Effects Of Cocaine
The acute effect of cocaine is to act at monoamine transporter proteins to block the reuptake of 5-HT, noradrenaline, and dopamine. Through the inhibition of monoamine reuptake, cocaine has three general effects on synaptic transmission (figure 1). First, the duration of monoamine- mediated synaptic potentials is dramatically prolonged. Second, monoamine autoreceptor activation is augmented to inhibit further release of monoamines. Third, heterosynaptic modulation of nonmonoamine transmitter release mediated by presynaptic monoamine receptors is augmented. Each of these actions of cocaine has been observed in brain slice experiments using the ventral tegmental area (VTA). It is interesting to note that all of the observations made in the VTA using low concentrations of cocaine, when applied acutely, center on the inhibition of the 5-HT transporter.
Neurons originating in the VTA are thought to play a key role in the formation of addictive behaviors. Thus, the effects of cocaine on the membrane properties and synaptic potentials on neurons in this area are critical to understanding the cellular effects of this drug. There are at least three different populations of VTA neurons identified by the different synaptic potentials and the response to dopamine, 5-HT, and opioids. The majority of cells (about 60 percent) were tyrosine hydroxylase positive and were hyperpolarized by dopamine. These cells had a slow GABAb-mediated inhibitory postsynaptic potential (IPSP) and are termed "principal cells." About 10 percent of cells, termed "secondary cells," were hyperpolarized by [Met]-5-enkephalin and failed to respond to dopamine. A third group of cells, "tertiary cells," were hyperpolarized by dopamine, 5-HT, and [Met]-5-enkephalin. These cells had a slow 5-HT1a-mediated IPSP (Cameron et al. 1997).
The 5-HT1a-mediated IPSP observed in tertiary cells in the VTA was very sensitive to cocaine. Low concentrations increased both the amplitude and prolonged the duration of this IPSP. Higher concentrations of cocaine further prolonged the duration but decreased the amplitude of the IPSP. These results indicate that the inhibition of 5-HT reuptake by cocaine has a dramatic effect on 5-HT-mediated synaptic transmission. The prolonged presence of 5-HT increased the duration of the IPSP and caused a feedback inhibition of further 5-HT release by an action on 5-HT1d receptors located on the 5-HT- releasing terminals. A third acute effect of cocaine was observed while recording from principal neurons in the VTA (Cameron and Williams 1994). Cocaine caused a concentration-dependent presynaptic inhibition of the GABAb-mediated IPSP. This inhibition was mimicked by exogenously applied 5-HT and the 5-HT releaser, fenfluramine (10 M). In addition, the inhibitions by cocaine, 5-HT, and fenfluramine were all antagonized by the nonselective 5-HT antagonist metergoline (1 M). To confirm that the action of cocaine was mediated through the inhibition of reuptake of endogenous 5-HT, slices were pretreated with parachloroamphetamine (PCA, 10 M, 2-4 hr) to deplete endogenous 5-HT. The effects of cocaine and fenfluramine but not sumatriptan were blocked in slices treated with PCA.
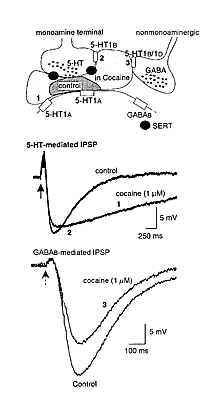 |
Figure 1. Cocaine dramatically changes monoamine- mediated synaptic transmission. At the top is a schematic that shows a monoamine (5-HT) terminal that releases 5-HT onto a postsynaptic cell having 5-HT1a receptors (1). The monoamine terminal has both the serotonin transport protein (SERT) and 5-HT1b/1d autoreceptors that inhibit 5-HT release (2). The schematic also shows a nonmonoamine terminal that has 5-HT1b/1d receptors that inhibit the release of GABA (3). The darker shaded area indicates the area over which 5-HT diffuses following release under control conditions. The lighter shaded area indicates that the spread of 5-HT is dramatically increased following the blockade of the SERT with cocaine. The traces in the middle are two superimposed voltage recordings of 5-HT-mediated IPSPs from a tertiary cell in the VTA. The IPSP recorded in the presence of cocaine is longer lasting (1) and smaller (2) than in control. The traces at the bottom are GABAb- mediated IPSPs recorded in a dopamine cell in the VTA. In this case cocaine decreased the amplitude of the GABAb IPSP without changing the timecourse. This effect results from a presynaptic inhibition of GABA release caused by the activation of 5-HT1b/1d receptors located on the GABA-releasing terminal (3). |
Thus, one more effect of low concentrations of cocaine in the VTA is to inhibit GABA release. This effect of cocaine would be expected to result in disinhibition of dopamine cells to increase the release of dopamine.
Cocaine therefore has at least three actions on synaptic transmission in the VTA. Cocaine augments 5-HT-mediated IPSPs, augments autoreceptor-mediated inhibition of 5-HT release, and facilitates heterosynaptic inhibition of GABA release mediated by 5-HT1d receptors. Although not discussed here, similar mechanisms have been either demonstrated or predicted to occur at many sites in the CNS for each of the monoamine transmitters.
Adaptations Resulting From Chronic Cocaine Treatment
The mesolimbic system is known to play a role in self-administration of many drugs of abuse, including cocaine and opioids. Although morphine and cocaine act by separate cellular mechanisms initially, there must be a common pathway that results in the activation of reward pathways. One way to examine the potential common effects is to identify the common adaptations that result from repeated administration of drugs of abuse. Following chronic treatment with cocaine or morphine, a common change in synaptic regulation of dopamine cells in the VTA was observed 1 week after termination of chronic treatment. Normally D1 receptor activation augmented the amplitude of a GABAb IPSP (Cameron and Williams 1993), but in drug-experienced animals, D1 receptor activation caused an inhibition of the GABAb IPSP (Bonci and Williams 1996). The inhibition was blocked by adenosine A1-receptor antagonists and agents that disrupted the metabolism of cAMP. Thus, it appears that there is a long-lasting change in the balance between the augmentation of GABA release caused by D1-receptor activation and the inhibition mediated by adenosine that is produced by the metabolism of cAMP released from the GABA-containing nerve terminals that project from the nucleus accumbens or ventral pallidum.
This long-lasting dopamine/adenosine interaction may be one mechanism involved in dopamine-mediated craving and relapse to drug-seeking behaviors. This study suggests that neurochemical mechanisms that may be unrelated to the initial action of cocaine on the dopamine system, such as the augmentation of adenosine tone, can result in a persistent change in the synaptic regulation of dopamine cell activity.
Acknowledgment
This research was supported by National Institute on Drug Abuse Grant No. DA-04523.
References
Bonci, A., and Williams, J.T. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron 16:631-639, 1996.
Cameron, D.L.; Wessendorf, M.W.; and Williams, J.T. A subset of ventral tegmental area neurons is inhibited by dopamine,
5-hydroxytryptamine and opioids. Neuroscience 77:155-166, 1997.
Cameron, D.L., and Williams, J.T. Dopamine D1 receptors facilitate transmitter release. Nature 366:344-347, 1993.
Cameron, D.L., and Williams, J.T. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci 14:6763- 6767, 1994.
[NIDA Home] [Contents] [Next Section] [Previous Section]
|