Cocaine and the Changing Brain
Whole-Cell Plasticity In Cocaine Addiction: Current Perspectives
Francis J. White, Ph.D.
Finch University of Health Sciences
The Chicago Medical School
North Chicago, IL
Introduction
Cocaine addiction remains one of the foremost public health problems in the United States. Cocaine dependence is typically associated with cyclical patterns of drug use and abstinence. During abstinence, periods of intense cocaine craving and other withdrawal symptoms (anergia, anhedonia, and depression) often lead to relapse. Animal models of cocaine dependence have successfully identified several neuroadaptations in brain circuitry involved in cocaine addiction, particularly within the dopamine (DA) neuronal system projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and related limbic and cortical areas. These neuroadaptations range from changes in transporters, receptors, and transduction molecules to alterations in gene expression. Despite our increasing knowledge, pharmacotherapies targeted at such changes have yet to prove effective in cocaine-dependent individuals, in part because of our incomplete understanding of the relevant alterations and how they occur.
We have used electrophysiological techniques to study alterations in the mesoaccumbens DA system at the level of single neurons, particularly within the NAc. We have focused on the NAc because of its known role in cocaine self-administration and other forms of motivated behavior. NAc neurons receive convergent excitatory commands from the ventral hippocampus (subiculum), basolateral amygdala, and prefrontal cortex, which together supply information regarding environmental conditions, behavioral contingencies, emotional states, and motivational (homeostatic) needs. DA from the VTA neurons modulates such information by using a rich repertoire of presynaptic and postsynaptic mechanisms.
Nucleus Accumbens Neurons
A great deal of information regarding NAc neurons has emerged over the past half decade. The major neuronal population in the NAc is the medium spiny GABAergic output neuron. These neurons encode excitatory commands and relay information to two nuclei of the basal ganglia, the ventral (subcommisural) pallidum and the substantia nigra pars reticulata, as well as to the medial-dorsal thalamic nucleus, creating a loop back to the cortex. Like their neighbors in the caudate nucleus, medium spiny neurons of the NAc exhibit a two-state spontaneous membrane potential, fluctuating between a highly hyperpolarized state (-75 to -90 mV, the down-state) and a more depolarized state (-45 to -60 mV, the up-state) close to the firing threshold. The down-state represents periods of relatively little excitatory input and is maintained by an inwardly rectifying K+ conductance. Transition to the up-state and to spike activation requires a convergent barrage of maintained excitation from hippocampus, amygdala, and/or prefrontal cortex, with hippocampal information playing a strategic "gating" role for spike activity (O'Donnell and Grace 1995). However, maintenance of the up-state membrane potential requires interplay between several voltage-dependent inward and outward currents, which are subject to neuromodulation. Given the importance of such conductances in controlling the state of activity of NAc output neurons, we decided to pursue a program of study designed to characterize the modulation of various voltage-dependent channels, particularly by DA receptors, and to determine whether the channels and their modulation are altered by repeated cocaine administration.
Studies In Brain Slices
Our first experiments were conducted in brain slices. In this in vitro preparation, medium spiny NAc neurons exist only in the down-state because most excitatory afferents are severed. We used traditional current-clamp intracellular recordings to compare the membrane properties of NAc neurons in rats that received once-daily cocaine (15 mg/kg) or saline (control) injections for 5 days. All experiments were conducted on the third day after the last injection of cocaine. We found that the resting membrane potentials of cocaine-withdrawn NAc neurons were more hyperpolarized compared with control neurons. When we injected depolarizing current into the neurons to switch them to the up-state and induce action potentials (Na+ spikes), we found that cocaine- withdrawn neurons required significantly greater currents to evoke spikes. In addition, the threshold for producing action potentials was significantly increased, and the spikes were of smaller amplitude (see table 1). With sustained depolarization, control neurons exhibited repetitive firing, with the number of spikes increasing as a function of the intensity of current applied. Repetitive firing was either completely absent or markedly reduced in cocaine-withdrawn neurons. When voltage-sensitive sodium channels (VSSCs) were blocked by tetrodotoxin (TTX), we found that calcium plateau potentials were also reduced in both amplitude and duration. None of these alterations was observed in rats that received repeated administration of lidocaine, indicating that they were not the result of local anesthetic actions of cocaine.
TABLE 1. Repeated Cocaine Administration In Vivo Alters Membrane Properties of NAc Neurons Recorded In Vitro.
| Measures |
Saline |
Cocaine |
Lidocaine |
| Number of neurons |
36 |
29 |
14 |
| Number of rats |
25 |
19 |
4 |
| RMP (mV) |
-79.1 ± 0.8 |
-83.8 ± 0.6** |
-76.9 ± 1.5 |
| Current to generate AP (nA) |
0.65 ± 0.03 |
0.96 ± 0.06** |
0.69 ± 0.09 |
| AP threshold (mV) |
-46.7 ± 1.5 |
-40.9 ± 1.7* |
-46.1 ± 2.1 |
| AP amplitude (mV) |
61.1 ± 1.1 |
52.5 ± 1.7** |
59.2 ± 1.2 |
NOTE: Values represent the mean ± SEM for the number of neurons indicated (* p < 0.05, ** p less than 0.01 with Student's t-test used to compare with control). Resting membrane potential (RMP) was measured in the absence of injected current prior to the initiation of other manipulations. Action potentials (APs) were generated by injecting step depolarizing current pulses of 0.1 nA increments (adapted from Zhang et al. 1998).
Studies In Dissociated Neurons
The results of our brain slice experiments clearly indicated a reduction in the excitability of NAc neurons from cocaine-withdrawn rats and suggested alterations in voltage-sensitive channels. Therefore, we initiated a series of studies to evaluate whether various channels are altered by repeated cocaine administration. To date, we have characterized VSSCs and voltage-sensitive calcium channels (VSCCs). These experiments were conducted in acutely dissociated neurons using the whole-cell configuration of the patch-clamp technique and specific agents to block all but the channels of interest. This preparation afforded us considerable control in voltage-clamp mode without concerns of poor space-clamp and allowed us to conduct mechanistic studies of neuromodulation because we had access to the internal milieu of the neurons via the patch pipette.
We first demonstrated that, as in the caudate nucleus (Surmeier and Kitai 1994), DA D1 receptors reduce whole-cell Na+ current in medium spiny neurons of the NAc. The reduction occurs because of activation of the adenylyl cyclase-cAMP transduction pathway causing enhanced phosphorylation of VSSCs by cAMP-dependent protein kinase (PKA). In cocaine-withdrawn neurons, we observed a 37-percent reduction in Na+ current density and a depolarizing shift (of about 5 mV) in the voltage dependence of activation, with no alteration in the voltage dependence of inactivation (see figure 1). This profile of effects on VSSCs has been suggested by some investigators to indicate enhanced phosphorylation by protein kinase C (PKC). However, we found that activation of PKC by phorbol esters reduced Na+ current and delayed inactivation but did not alter the voltage dependence of activation. Thus, we interpret the reduction in basal Na+ current produced by repeated cocaine administration as an indication of enhanced basal state of phosphorylation by PKA because the cAMP-PKA pathway in the NAc is clearly upregulated by repeated cocaine treatment, with increases in levels of both cAMP and PKA (Terwilliger et al. 1991). Additional study will be required to elucidate the mechanism underlying the depolarizing shift in the voltage dependence of activation. Whatever the mechanism, this result is consistent with those obtained in brain slices (above), as well as those observed in vivo (White et al. 1995), all of which indicate a reduced excitability of NAc neurons during cocaine withdrawal (Zhang et al. 1998).
Our next series of experiments examined the effects of repeated cocaine administration on isolated VSCCs. Studies of modulation of VSCCs in medium spiny neurons of the NAc yielded results that again were highly similar to those previously reported for the caudate nucleus (Surmeier and Kitai 1994). DA D1 receptors suppressed whole-cell Ca2+ currents, particularly those carried by N and P/Q type channels. This modulation occurred via cAMP-PKA-protein phosphatase cascade leading to dephosphorylation of the channels and a reduction in current. After repeated cocaine administration, the basal level of whole-cell Ca2+ current was significantly reduced, and the N-type channel appeared to be most affected. Because these channels are primarily involved in transmitter release, a reduction in current would result in reduced GABA release from these neurons when they are active.
Conclusions
Our studies have demonstrated a novel form of whole-cell (nonsynaptic) plasticity produced by repeated cocaine administration reduced Na+ and Ca2+ currents in NAc neurons. This nonsynaptic plasticity should be considered an important contributor to behavioral manifestations of cocaine addiction and withdrawal. Consider that, while synaptic plasticity will modify the responsiveness of NAc neurons to selected inputs at specific synapses, the reduction of whole-cell Na2+ currents will produce an indiscriminate decrease in the responsiveness of the NAc to excitatory commands because these channels govern the initiation of action potentials. Even when activated, the neurons are likely to transmit less information to their targets because of the reduction of GABA release due to reduced Ca2+ currents. In addition, reduced Na+ and Ca2+ conductance could also impact synaptic plasticity (such as long-term potentiation or depression), because VSSCs expressed within neuronal soma and dendrites carry backpropagating action potentials that modulate dendritic Ca2+ influx. Thus, even if whole-cell plasticity does not persist with longer withdrawal, it will help to determine which synapses undergo long-term associative synaptic plasticity as well as the magnitude of such changes.
| Figure 1. Repeated cocaine treatment reduced NAc Na+ currents and caused a depolarizing shift in the voltage dependence of activation. |
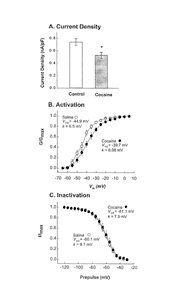 |
A. NAc neurons (N = 33) from cocaine- pretreated rats exhibited significantly (t68 = 2.75, p = 0.0075) reduced peak whole- cell Na+ currents (measured at -20 mV) compared with neurons (N = 37) obtained from saline-pretreated rats.
B. Cocaine pretreatment caused a depolarizing shift in the voltage dependence of activation and reduced the slope factor (k). Values were obtained for each neuron at each membrane voltage, and the means ±SEM were plotted. Half maximal activation (V1/2) and slope (k) values were obtained individually for each neuron and were compared with t-tests. Half-maximal activation occurred at significantly (t28 = 2.26, p = 0.031) more depolarized potentials in cocaine-pretreated neurons (-40.3 ± 1.8 mV, N = 16) compared with saline-pretreated neurons (46.1 ± 1.2, N = 14). The slope factor was also significantly decreased (t28 = 3.13, p = 0.004) in the cocaine group (8.85 ± 0.8) compared with the saline group (4.01 ± 0.8). Note that the V1/2 and k values shown in the figure were derived from the curves fitted to the mean values depicted in the figure, not from the mean values (given here) obtained by averaging all of the neurons in the sample.
C. The voltage dependence of inactivation was not altered by repeated cocaine treatment. Values were obtained from the means ± SEM as in B (N = 16 for cocaine and 13 for saline). Note that repeated administration of lidocaine failed to produce effects similar to those of cocaine (adapted from Zhang et al. 1998).
|
Given that the NAc is a structure in which functionally distinct ensembles of neurons are recruited by convergent excitatory inputs to coordinate patterns of movement and affect, a reduction in the excitability of NAc neurons would decrease the processing of such information and thereby lead to cocaine withdrawal effects such as anergia, anhedonia, and depression. In rat models, such deficits are observed during the early days of withdrawal from repeated cocaine administration when Na+ and Ca2+ currents are depressed. Moreover, given that D1 receptor stimulation further reduces both Na+ and Ca2+ currents in the cocaine-withdrawn NAc neurons, we believe that the proposed use of such drugs as a replacement therapy for cocaine-dependent individuals may be contraindicated, at least during the early period of cocaine withdrawal.
Acknowledgments
This research was supported by National Institute on Drug Abuse Grant No. DA-04093 and Research Scientist Development Award DA-00207.
References
O'Donnell, P., and Grace, A.A. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: Hippocampal gating of prefrontal cortical input. J Neurosci 15:3622-3639, 1995.
Surmeier, D.J., and Kitai, S.T. Dopaminergic regulation of striatal efferent pathways. Curr Opin Neurobiol 4:915-919, 1994.
Terwilliger, R.; Beitner-Johnson, D.; Sevarino, K.A.; Crain, S.M.; and Nestler, E.J. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res 548:100-110, 1991.
White, F.J.; Hu, X-T.; Zhang, X-F.; and Wolf, M.E. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther 273:445-454, 1995.
Zhang, X-F.; Hu, X-T.; and White, F.J. Whole-cell plasticity in cocaine withdrawal: Reduced sodium currents in nucleus accumbens neurons. J Neurosci 18:488-498, 1998.
[NIDA Home] [Contents] [Next Section] [Previous Section]
|