News and Events
Congressional Justification for FY 2005
National Eye Institute
Authorizing Legislation:
Section 301 and Title IV of the Public Health Service Act, as amended. Preauthorizing legislation will be proposed.
Budget Authority:
| FY2003 Actual | FY2004 Final Conference | FY2005 Estimate | Increase or Decrease | ||||
| FTE | BA | FTE | BA | FTE | BA | FTE | BA |
| 237 | $632,268,000 | 227 | $652,738,000 | 227 | $671,578,000 | 0 | $18,840,000 |
This document provides justification for the FY 2005 activities of the National Eye Institute, including HIV/AIDS activities. A detailed description of the NIH-wide FY 2005 HIV/AIDS activities can be found in the NIH section entitled "Office of AIDS Research (OAR)."
- Story of Discovery
- Science Advances and Future Research Directions
- NIH Roadmap
- NEI New Initiatives
- Innovations in Management and Administration
- Budget Policy
Introduction
Congress created the National Eye Institute (NEI) with the mission to conduct and support research, training, health information dissemination, and other programs with respect to blinding eye diseases, visual disorders, mechanisms of visual function, preservation of sight, and the special health problems and requirements of individuals who are visually impaired. Inherent in this mission is clinical research across the spectrum of diseases of the eye and disorders of vision, as well as the investigation of the normal tissue and normal visual processes that will help gain a more complete understanding of the abnormal processes that lead to these conditions. These investigations are conducted in hundreds of extramural laboratories and clinics throughout the United States and in the NEI's own intramural facilities in Bethesda, Maryland. The highlights that follow are examples of the research progress that has been made with the investment of Federal funds in NEI-supported research and the direction that research will take over the next year.
Story of DiscoveryNobel Prize Winning Research on the Physiology of Water Movement in Cells. The clinical relevance of research on fundamental cellular processes may require many years of work before the knowledge is medically useful. Among the most fundamental physiological processes essential for life are the mechanisms used by cells, tissues, and organs of the body to manage water content and flow. Water movement in tissues requires its transport across the cell membrane. Strict regulation of cellular salt and water content is essential for virtually all metabolic activity, so the cell membrane must manage the transport of these substances into and out of the cell. By 1935, it was suggested that proteins embedded within the cell membrane were responsible for a variety of cellular functions including sodium and water flow. The following decades of research demonstrated that transport of salts and small organic molecules, such as glucose, is accomplished via two types of membrane proteins: channels, which permit rapid flow via diffusion through molecular sized pores; and carriers, which bind the transported molecules and shuttle them across the membrane. Although its existence was predicted more than 50 years earlier, a protein specialized for water transport across membranes remained an elusive target. In 1983, various transport proteins were proposed to double as the putative water channel, but none were demonstrated experimentally. Then in 1992, Dr. Peter Agre discovered a small, abundant protein in red blood cell membranes that also was identified in kidney tubules. Subsequent isolation, purification, and insertion of this protein into membranes confirmed its function as a water channel, and it later was named aquaporin-1. Dr. Agre established that aquaporin-1 (AQP1) is one member of a family of ten mammalian aquaporins, and hundreds of similar proteins have been identified in virtually all forms of life. His work has progressed rapidly as knowledge of the specific cellular locations of the various forms of aquaporins has generated a more accurate picture of how water transport is maintained and regulated in tissues that have high rates of fluid transport, such as kidney, and the salivary, lacrimal and sweat glands, as well as those tissues that must maintain strict regulation of water content, such as the brain, the cornea and lens of the eye, and the respiratory tract. Clinician scientists are beginning to identify a multitude of diseases that are associated with genetic defects or altered regulation of various aquaporins. The identification and localization of aquaporin family members has revealed the complexity that has evolved to manage salt and water membrane permeability. Future research will continue to explore the regulatory pathways that control these important transporters as well as their function. Furthermore, scientists have just begun to identify the role of aquaporins involved in a number of diseases, e.g. Sjögren's syndrome, an autoimmune disease that drastically reduces tear flow, some kidney diseases, congestive heart failure, and cirrhosis. Understanding how their function is disrupted will lead to new therapeutic targets. For his work Dr. Agre, an NIH and NEI grantee who has led much of the effort to understand aquaporins, shared the 2003 Nobel Prize in chemistry with another NIH grantee, Dr. Roderick MacKinnon. |
Science Advances and Future Research Directions
Retinal Diseases
The retina is the complex, light-sensitive, neural tissue in the back of the eye that contains highly-specialized and metabolically active photoreceptor cells (rods and cones). These cells respond to light by emitting chemical and electrical signals. These signals are received by other retinal cells that process and transmit visual information via the optic nerve to the brain for further processing. The choroid is the underlying layer of blood vessels that nourish the retina. The retina and choroid are susceptible to a variety of diseases that can lead to visual loss or complete blindness. These sight-threatening conditions include age-related macular degeneration, diabetic retinopathy, retinopathy of prematurity, retinitis pigmentosa, Usher's syndrome, ocular albinism, retinal detachment, uveitis (inflammation), and cancer (choroidal melanoma and retinoblastoma).
Early Treatment for Retinopathy of Prematurity (ETROP) Study. Retinopathy of prematurity (ROP) is a disease of the eyes of very low birthweight premature infants in which the retinal blood vessels increase in number and branch excessively, sometimes leading to hemorrhage or scarring that can result in vision loss or blindness. In the late 1980s, a NEI-supported clinical trial known as the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) Study demonstrated the safety and efficacy of freezing a portion of the peripheral retina (cryotherapy) in preventing progression of the disease and subsequent vision loss or blindness. The ETROP Study was designed to test the hypothesis that earlier treatment in carefully selected cases will result in an overall better visual outcome than treatment at the conventional CRYO-ROP threshold for treatment of the disease. Researchers recently reported that premature infants at the highest risk for developing vision loss from ROP will retain better vision when therapy is administered at high-risk prethreshold. This treatment approach was found to be better than waiting until ROP has reached the traditional treatment threshold. The study also established the value of an improved risk assessment model to more accurately identify those infants who are at the highest risk for developing severe vision loss from ROP. Results also clearly indicate that for certain subgroups of eyes, watchful waiting is the best approach.
Long-term Follow-up of Diabetic Retinopathy Treatment. Diabetic retinopathy is a leading cause of vision impairment in the adult population in the United States. The treatment strategies for diabetic retinopathy developed over the last several decades are, in part, based on NEI-supported clinical trials evaluating diabetes control, laser photocoagulation, and vitrectomy. Although these treatments were found to be 90 percent successful in reducing the risk of severe vision loss over short term, the long term effects of these treatment strategies were not known. A follow-up study of Early Treatment Diabetic Retinopathy Study patients from one local treatment center was conducted at the National Eye Institute, 13 to 19.5 years after the initial laser photocoagulation (median, 16.7 years). Although the mortality rate of patients with diabetic retinopathy is much higher than that of the general population, clinical scientists found that aggressive follow-up, with treatment when indicated, seems to be associated with maintenance of good long-term visual acuity for most patients. Vigilant follow-up is essential in maintaining good vision in patients with diabetic retinopathy. This underscores the need to increase screening for diabetic retinopathy and the delivery of timely treatment in patients with diabetes.
Avoiding Advanced Age-related Macular Degeneration (AMD) and Vision Loss. AMD is the leading cause of blindness in patients over age 60 in the U.S. and is a major health problem in most other developed countries. An estimated 8 million Americans at least 55 years old are at high risk to develop advanced AMD. Based on the results of the Age-Related Eye Diseases Study (AREDS), 1.3 million of these people would develop advanced AMD if no treatment were given to reduce their risk. If these people at risk for development of advanced AMD received the supplements (vitamins C, E, beta-carotene, and zinc) used in AREDS, more than 300,000 of them would avoid advanced AMD and any associated vision loss over the next 5 years.
Programmed Cell Death in Age Related Macular Degeneration. Similar to neuronal cells in the brain, the cells in the retinal pigment epithelium (RPE) are non-dividing cells that are integral to maintaining a number of physiologic functions important to vision. These are the main cells involved in AMD, but the mechanisms involved in the RPE programmed cell death or apoptosis are unknown. Most investigations of programmed cell death have focused on certain molecules called caspases that initiate a series of events that lead to DNA damage and cell death. While this caspase-dependent system is clearly involved in many cell types, NEI intramural researchers have demonstrated conclusively that the caspase system in not involved in RPE programmed cell death. By using a variety of molecular and cellular techniques, they found that apoptosis-inducing factor was the primary protein involved in RPE cell death. These scientists also found that treatment of RPE cells with a certain growth factor, hepatocyte growth factor/scatter factor, prevented cell death. This is the first observation suggesting a potential therapy for the geographic form of AMD. Therefore, continuing this research toward developing an effective treatment for this condition would have significant positive health implications and would potentially reduce the financial burden of disability costs for these patients.
Humanized Anti-Interleukin-2 (IL-2) Receptor Alpha Therapy. Uveitis is an autoimmune inflammatory disease of the eye that accounts for up to 10 percent of blindness in the U.S. It is believed that most of the severe inflammations of the eye seen in this country are non-infectious in origin. In collaboration with researchers at NCI, NEI intramural scientists used a monoclonal antibody (daclizumab) as a treatment for experimentally induced autoimmune uveitis in nonhuman primates and found that the treatment had a positive therapeutic effect. Researchers have now reported the results of use of this monoclonal antibody in the long term treatment of patients with uveitis. Patients who previously needed systemic immunosuppression to control their uveitis could be taken off of this standard therapy and maintained only with the antibody therapy given at monthly intervals. This new therapy may permit long-term treatment of patients with severe uveitis with many fewer side effects than existing therapies, leading to an improved quality of life. Further, these results have led others to adopt this treatment strategy with particularly promising results in multiple sclerosis patients. Planning is underway to begin a Phase III study to evaluate the full potential use of this therapy.
Mitochondrial Gene Therapy for Leber's Hereditary Optic Neuropathy. Leber's Hereditary Optic Neuropathy (LHON) is an inherited disease characterized by sudden visual failure in individuals between 20 and 30 years of age. Half of these affected individuals will become totally blind. Ninety-five percent of these cases can be linked to three different DNA mutations in mitochondrial DNA rather than in the nuclear DNA that codes for most proteins. Mitochondrial DNA codes for 13 proteins that are essential for the energy production required for metabolic processes in living organisms. The repair of mitochondrial DNA is a special challenge, because it is not possible to directly incorporate new DNA and replicate it in mitochondria to repair mutations. Mitochondrial and nuclear DNA and protein are also located in different regions of the cell and have differences in their sequences. Researchers supported by the NEI were able to overcome these and other biological roadblocks to effect the first rescue of a LHON gene mutation in a model system by engineering mitochondrial DNA to produce the correct protein in the nucleus. The protein was then successfully incorporated into the mitochondria resulting in normal metabolic functions. Future research will focus on understanding the pathophysiology of LHON to provide more precise tools for a gene-based treatment of this disorder in patients.
RNA Interference Gene Therapy. NEI-supported researchers are exploring the use of a new gene therapy approach, called RNA interference, in treating eye disease. In nature, organisms are equipped with genetic sequences that regulate gene expression to insure that a cell encodes the required quantities of a protein needed to function and survive. If a gene produces too much of a protein, it can have a detrimental affect. RNA interference is a naturally occurring biological process that cells employ to control the expression of genes. When a gene initiates the production of a protein, it releases a copy of the coding sequence from the nucleus of the cell. The cell then reads the sequence, called messenger RNA (ribonucleic acid), to construct a protein. RNA interference causes destruction of the messenger RNA, thus preventing the production of the protein. Borrowing from nature, NEI-supported researchers have recently developed synthetic RNA interference sequences to prevent the expression of a gene for vascular endothelial growth factor (VEGF). As its name implies, VEGF spurs blood vessel growth. NEI-supported researchers have previously found increased protein levels of VEGF in patients with macular degeneration and diabetic retinopathy. In animal models of disease, RNA interference blocked VEGF gene expression and prevented the growth of blood vessels. Continuation of this line of research not only has implications for treatment of a variety of diseases, but it will also help researchers to gain insight into a gene's function in health and disease and study its role in development.
Corneal Diseases
The cornea is the transparent tissue at the front of the eye that serves two specialized functions. The cornea forms a protective physical barrier that shields the eye from the external environment. It also serves as the main refractive element of the eye, directing incoming light onto the lens. Refraction depends on the cornea acquiring transparency during development and maintaining this transparency throughout adult life. Corneal disease and injuries are the leading cause of visits to eyecare clinicians, and are some of the most painful ocular disorders. In addition, approximately 25 percent of the American population have a refractive error known as myopia or nearsightedness that requires correction to achieve sharp vision1; many others are far-sighted or have astigmatism.
The Cornea Eye Has Special UV Protection Mechanisms. The cornea must remain transparent for light to enter the eye and for images to be properly focused on the light-sensitive retina. Despite its exposure to cancer-causing UV radiation, the cornea rarely develops primary tumors. The cornea has developed a unique mechanism to transport an important DNA protective molecule into its nucleus. Although most cells have high cytoplasmic levels of the protein ferritin, corneal epithelial cells have high levels of ferritin in the nucleus. In this location, ferritin is highly effective in protecting DNA from UV-induced damage. Proteins are synthesized in the cytoplasm of the cell. To move from the cytoplasm to the nucleus, substances must pass through holes or pores in the nuclear membrane. Large proteins that are too big to move through the pore by diffusion contain nuclear localization sequences that facilitate passage. Ferritin does not possess these sequences but researchers have discovered a novel protein called ferritoid, specific for corneal epithelial cells, which binds to ferritin and carries it into the nucleus. This work may provide a model for developing other molecules that could serve to shuttle other protective substances to the nucleus that could also be designed specifically for certain cell types. More knowledge about the mechanism of action of ferritoid may also provide clues for how the nuclear pore complex operates and may permit studies of ferritin's mechanism of action after penetrating the nuclear membrane.
Exploration of Innovative New Drug Delivery Systems in the Cornea. Topical drug delivery for the treatment of diseases and disorders of the cornea is generally limited to small lipophilic (lipid soluble) agents that are only partially absorbed because of the corneal barrier function of the epithelium and tear drainage. NEI intramural scientists have used a mouse experimental model to study a more efficient means of drug delivery to the cornea. These scientists found that serum albumin represents up to 13 percent of the total water-soluble protein of the mouse cornea. Humans also have abundant serum albumin in the corneal stroma. Because the serum albumin accumulates in the corneal stroma by diffusion from the blood supply surrounding the cornea, it may provide an alternative route of drug delivery to the cornea. Conjugating serum albumin to the drug of choice and injecting the conjugate into the blood stream will not only direct the drug within the cornea, but extend its half-life within this tissue. Moreover, the use of a photolytic bond to conjugate the drug to the serum albumin will allow drug release in the transparent cornea upon exposure to light. Future research using this mouse model will allow researchers to test the usefulness of serum albumin as a drug carrier to treat corneal disorders.
The Immunology of Herpes Latency. Ocular Herpes simplex virus (HSV) infection is the most frequent serious viral eye infection and the leading cause of viral induced corneal blindness in the United States. The virus persists indefinitely in infected individuals. Up to 90 percent of adults possess circulating antibodies against the virus. HSV causes a variety of diseases, including herpetic stomal keratitis in the cornea, as well as genital, brain, and skin afflictions. Subsequent to a primary infection, HSV establishes lifelong latent infection in nerve ganglia, followed by episodes of periodic reactivation. The long accepted explanation is that HSV persistence is due to the expression of viral latency-associated transcripts, which do not appear to encode any antigenic components. It is assumed that this absence of antigen expression allows the virus to escape recognition by the host immune system. Researchers showed that immune T-cells in latently infected ganglia are specific for HSV and are critical for regulation of HSV latency by inhibiting viral reactivation. Their research indicated that there is a dynamic balance between HSV-1 latency and reactivation involving a tripartite interaction among the virus, the host neuron, and local immune components. The findings of the host immune components in HSV latency should lead future HSV research in a new direction that will have a high impact in not only eye infection but a wide spectrum of human herpetic diseases.
Regulation of Corneal Wound Healing. Rapid healing of corneal epithelial wounds is necessary to maintain the optical clarity of the cornea and to provide a barrier to infection. Although wound healing is often impaired by diabetes or other conditions, effective treatments are limited by a lack of understanding of the underlying cellular mechanisms. NEI intramural scientists recently identified an enzyme called CDK5 that regulates corneal epithelial cell adhesion and migration. Although CDK5 is expressed primarily in neurons, it is also expressed in many non-neuronal tissues, including ocular epithelial tissues such as the corneal epithelium and lens, where its function has been obscure. Using a model wound healing system, these researchers found that the rate of wound closure was significantly retarded in cells with too much CDK5 and accelerated in cells in which the CDK5 was inactivated. They concluded that CDK5 is an important regulator of corneal epithelial cell adhesion and migration and may play a significant role in the rate of corneal epithelial wound healing. Continuation of this line of research may provide the means to promote the healing of corneal tissues that have been damaged by disease or injury.
Cataract
Cataract, an opacity of the lens of the eye, interferes with vision and is the leading cause of blindness in developing countries. In the U.S., cataract is also a major public health problem. The enormous economic burden of cataract will worsen significantly in coming decades as the American population ages. The major goals of this program, therefore, are to determine the causes and mechanisms of cataract formation, to search for ways to slow or prevent the progression of cataract, and to develop and evaluate new diagnostic and therapeutic techniques in cataract management.
Small Heat Shock Proteins Key to Lens Transparency in Aging. Age-related cataract formation is believed to result from the complex effects of aging on normal physiological processes. It has long been recognized that lens transparency is a function of a very high concentration of soluble proteins, the crystallins, within the specialized lens fiber cell. Previous studies have shown the steps involved in α-crystallin binding and stabilization of its target proteins. Like other small heat shock proteins that react to cellular stress, α-crystallin has some flexibility in function depending on the cell environment. New data suggest that under low stress, α-crystallin is maintained in a multi-subunit complex. Under conditions of high stress, α-crystallin breaks into smaller sub-units. This shift coincides with the formation of a high capacity form increasing the efficiency of the α-crystallin chaperone function due to an increase in surface binding capacity. This chaperone function protects against clouding of the lens due to protein aggregation. Studies from around the world in the last 15 years have shown α-crystallins are used for many important biological processes, including regulation of cell division and cell death and organization of the cellular skeleton, which is critical for the shape and movement of cells. In the lens α-crystallin has a dual function: it accumulates in fiber cells in high concentrations to produce the high refractive index needed for transparency, and it functions as a molecular chaperone. Improving our understanding of this protective role of α-crystallin may one day lead to the means to prevent cataract.
Understanding Lens Cell Communication. The lens contains two cell types: metabolically active epithelial cells and quiescent fiber cells. Throughout life, the lens continues to grow, with epithelial cells dividing and differentiating into fiber cells. During differentiation epithelial cells lose their intracellular metabolic structures and accumulate high concentrations of crystallin proteins to produce the high refractive index required for transparency. Because fiber cells lack the infrastructure for metabolism, they depend on epithelial cells for sustenance. The lens utilizes gap junctions, proteins in the cell membrane that allow the exchange of small molecules, to meet their unique requirements. Gap junctional communication joins the cells of the lens into a functional unit in which the metabolically active epithelial cells can control the environment needed in the fiber cells to maintain transparency. Mutations in genes encoding gap junction proteins that lead to dysfunctional proteins result in hereditary cataracts. Two different types of gap junction proteins known as connexins exist in the lens. Scientists have found that mice with deletions in genes encoding connexins develop cataracts as well as other developmental abnormalities. These studies have revealed differing roles for the two gap junction proteins, one is primarily responsible for maintaining transparency and the other is needed for growth. Researchers also found that a mutation in the growth associated gene resulted in hereditary cataracts in humans. These studies highlight the significance and importance of future research on the intricate communication system of the lens for maintaining lens health and transparency.
Glaucoma
Glaucoma is a group of eye disorders that share a distinct type of optic nerve damage, which can lead to blindness. Elevated intraocular pressure is frequently, but not always, associated with glaucoma. Glaucoma is a major public health problem and the number one cause of blindness in African Americans. Approximately 2.2 million Americans have been diagnosed with glaucoma and a similar number are unaware that they have the disease.2 Most of these cases can be attributed to primary open angle glaucoma, an age-related form of the disease. NEI activities in glaucoma research are directed toward understanding the mechanisms of the disease through basic research, identifying risk factors, and preventing blindness.
A Gene Discovery System for Glaucoma. Even though glaucoma was first described over 100 years ago, an explanation for its pathogenesis has eluded scientists. Elevated intraocular pressure is believed to be an important factor in the majority of cases of glaucoma. Elevated intraocular pressure results from an imbalance in the inflow and outflow of aqueous humor, the fluid that circulates in the front of the eye. Scientists have mapped and identified a gene associated with an inherited form of glaucoma. This gene encodes the protein myocilin, found in cells of the trabecular meshwork, a tissue involved in outflow of aqueous humor. Using myocilin as a tool, scientists genetically modified the common fruit fly, Drosophila melanogaster, to over-express the myocilin protein. The mutants had enlarged eyes that indicated an imbalance between aqueous humor production and uptake. The scientists then compared the genes expressed in the eyes of mutant flies to their normal counterparts. A number of genes were found to be altered, including several that had already been linked to glaucoma in humans. In order to validate their findings, the investigators used an organ culture created from donated human eyes. They found that one of the gene products, whose expression was enhanced in flies over-expressing myocilin, was also overproduced in the human system. Although glaucoma research lacks a genomic model system, scientists have been able to use Drosophila as a gene discovery system. Future research using this powerful tool may help identify potential proteins and physiological cascades that contribute to the disease, and may identify candidate genes to facilitate the mapping of disease genes in human patients.
Potential Neuroprotecive Therapy for Glaucoma. A hallmark of glaucoma is the death of ganglion cells in the retina, which can lead to catastrophic vision loss. Insights gained from a series of NEI-supported studies in animal models have recently culminated in an experimental gene therapy treatment for glaucoma that might one day augment or replace existing treatments to better protect retinal ganglion cells (RGCs). Previous NEI studies have found evidence that elevated intraocular pressure deprives RGCs of brain-derived neurotrophic factor (BDNF), an endogenous protein that is crucial to RGC survival. Although RGCs produce some BDNF, levels are further enhanced by adjacent cells of the lateral geniculate nucleus, which produce and transport BDNF to RGCs. Ocular injections of BDNF in rodent models of glaucoma have improved RGC survival. However, due to the relatively short half-life of this protein, the need for frequent ocular injections would not bode well in treating a chronic disease like glaucoma. To overcome this hurdle, NEI-supported researchers recently used gene therapy in rodent models of glaucoma to transfect RGCs with the gene that encodes BDNF, providing a lasting and direct supply of this essential protein. In short-term experiments, treated eyes had a marked improvement in RGC survival than those of control animals. Further NEI-supported laboratory work is evaluating whether gene therapy with BDNF provides long-term benefit and whether gene delivery with other neurotrophic agents, alone or in combination with BDNF, improves RGC survival.
Strabismus, Amblyopia, and Visual Processing
Developmental disorders such as strabismus (misalignment of the eyes) and amblyopia (commonly known as "lazy eye") affect 2-4 percent of the United States population3, 4. The correction of strabismus is one of the most frequently-performed surgical procedures. In addition to research relevant to strabismus and amblyopia, irregular eye movements, and refractive errors. Three million Americans now have chronic visual conditions that are not correctable by eye glasses or contact lenses (low vision)5. Therefore, the NEI also supports research on improving the quality of life of persons with visual impairments by helping them maximize the use of remaining vision and by devising aids to assist those without useful vision.
Better Treatment Options May Mean Better Compliance for Childhood's Most Common Eye Disorder. Amblyopia, or "lazy eye," is a common cause of monocular visual impairment, affecting 2 or 3 of every 100 children in the United States. Patching the stronger eye to force use of the weaker eye has been the mainstay of amblyopia therapy. Unfortunately, there is no specific patching regimen that is widely accepted for treating moderate amblyopia. Two patching regimens are commonly prescribed in clinical practice. One is minimal occlusion for 2 hours per day and the other is use of occlusion for 6 or more hours per day. Until recently, there were no data available to favor the use of one regimen over the other and compliance with patching varied widely. To address the clinical issue of the optimal number of patching hours for moderate amblyopia, a clinical trial comparing 2 hours versus 6 hours of daily patching for children with moderate amblyopia was conducted by the Pediatric Eye Disease Investigator Group (PEDIG), a network of eye care professionals involved in determining the best treatments for various eye problems in children. The results from this clinical trial revealed that patients in both groups showed substantial improvement in the eye with amblyopia. At 4 months, 79 percent of patients in the 2-hour patching group and 76 percent of patients in the 6-hour patching group had improved by two or more lines on the eye chart. Patching the unaffected eye of children with moderate amblyopia for 2 hours daily works as well as patching the eye for 6 hours.
In a parallel study conducted by PEDIG investigators, children with severe amblyopia who were less than 7 years of age were treated with either full-time patching (all waking hours or all but one) or 6 hours of patching in combination with one hour of near work like reading or coloring. Researchers found that 6 hours of patching with an hour of near work was as effective in improving visual acuity as full-time patching. Both of these studies should lead to better compliance with treatment for children with amblyopia. These results will change the way doctors treat moderate and severe amblyopia and make an immediate difference in treatment compliance and the quality of life for children with this eye disorder.
|
Story of Discovery Keeping Track of the Visual Scene: When we look at the car in front of us and then at the stop sign at the curb, there is a jump of the visual scene as our eyes are pointed toward the car and then to the sign. If we were shown a movie in which the car was immediately replaced by the sign, we would feel unsettled and be tempted to leave the movie. If the movie changed the scene two to three times per second, we would probably feel sick and leave the movie. But this is exactly what happens to our vision with normal eye movements. Scientists have puzzled over how we seem to be able to perceive a stable visual world in spite of this continual jumping about with each eye movement. One explanatory hypothesis has been that each time the brain sends out a command to move the eye, it sends a corollary or copy of that command to other regions of the brain to inform them that an eye movement is about to occur. The brain regions that receive information from the eye then know that it is about to move and to take into account this movement in interpreting the visual scene that is coming from the eye. The idea of a corollary discharge is an interesting one, but it has not been validated in primate brains. However, in recent experiments on the Rhesus monkey, NEI scientists believe that they have found this signal emanating from eye movement related areas in lower areas of the brain up to the cerebral cortex where much of the visual information is processed. They were able to demonstrate that by interrupting this pathway, the monkey was unable to take account of its own eye movement as it could when the pathway was functioning normally. Identification of this pathway opens the possibility for understanding how visual stability is maintained in spite of eye movements, and more generally for understanding how we keep track of all the movements that we are continually making. The understanding has important clinical implications as well, since impairments of corollary discharge have been hypothesized to contribute to a variety of neurological conditions, including Parkinson's disease and schizophrenia. |
Low Vision and Blindness Rehabilitation
Restoration of Vision. A team of NEI-supported visual scientists recently studied the rare case of sight restoration in an individual who had been blind since childhood, a period of almost 40 years. Although he had lost one eye and the sight in the other in an accident when he was 3½ years old, the vision in his remaining eye was recently restored through a corneal stem cell transplant. Because his vision was severely deprived during a large part of the critical period of visual development that normally ends at age 8, scientists were interested in determining what qualities of vision remained or what could be re-established. These researchers found the individual could indeed see, but the ability of his brain to integrate and understand what he sees was far from normal. The results of this study indicate that certain properties of vision, such as perception of color, shape, and motion, are established early in development and can survive to some degree even during an extended period of visual deprivation. Other complex features of vision, including the perception of complex objects, like faces or text, perception of the texture of a complex scene, perception in three dimensions and many other features that constitute vision, were absent or significantly reduced. Either the complex features of vision are not established early in visual development, or if they are, they need to be sustained by visual input. This research has provided a unique opportunity to examine how the brain adapts during a long period of visual deprivation and to increase our understanding of the critical period. Future research in this area will be vital to full restoration of vision in those people who regain vision subsequent to its loss during the critical period.
Technological Innovations
The Retinal Prosthesis Enters Phase One Clinical Trials. The marriage of computer technology and medical science is creating advances in treating even the most intractable diseases. In one such union, specially designed computer chips implanted in the eye may make it possible to restore some measure of visual function to the blind. Retinal degenerative diseases such as retinitis pigmentosa damage and destroy the light-sensitive photoreceptor cells in the retina. Although these cells die, much of the remaining nerve cell network in the retina remains healthy. The microelectronic retinal prosthesis, a device developed by researchers at the University of Southern California, mimics the function of photoreceptor cells by turning light into electric signals. The device consists of an implantable computer chip that receives signals from a camera mounted on a pair of glasses. In a recently published study, a 74 year-old patient who has been blind for more than 50 years from retinitis pigmentosa was implanted with a retinal prosthesis. The patient then underwent 10 weeks of visual assessment. Using the device, the patient was able to see spots of light, detect motion, and recognize simple shapes. When the prosthesis was turned off, the patient could not perform any of these tasks. Although preliminary, these results are a promising first step in realizing a prosthetic device that can restore ambulatory vision to patients with severe visual impairments due to retinal degenerative diseases, a major cause of vision loss in this country.
National Eye Health Education Program (NEHEP)
The NEHEP is a Partnership with over 65 national organizations in the private and public sector. Program areas of diabetic eye disease, glaucoma, and low vision were expanded to reach targeted high risk audiences particularly older adults, Hispanics and American Indians/Alaska Natives. The NEHEP Planning Committee recently met to begin development of a vision rehabilitation program for health professionals. The goal of this program is to ensure that patients are referred appropriately to available rehabilitation services.
NIH Roadmap
The NIH Roadmap was designed to identify major opportunities and gaps in biomedical research that could best be addressed as a concerted undertaking by the entire NIH. This shared effort and vision for a more efficient and productive system of medical research encompasses a number of areas of scientific opportunity that fall within the mission of the NEI. Some are areas in which the Institute is already investing, and some are areas in which the NEI is proposing initiatives. In nanomedicine, for example, the NEI is currently supporting grants that range from investigation of nanoparticle delivery of therapeutic agents for a variety of eye diseases to the development of nanoscale methods to stimulate membrane receptor proteins. Such methods may be useful in preserving the ability of neurons in retinas damaged by disease to transmit nerve signals. The NEI director has played and continues to play a key leadership role in developing and implementing the NIH nanomedicine initiative.
The NEI is also engaged in the roadmap efforts to re-engineer the clinical research enterprise. In its most recent strategic plan the NEI emphasized the need for application of the knowledge gained through research to benefit those who suffer from diseases of the eye or disorders of vision. The NEI emphasis on translational research has resulted in the Diabetic Retinopathy Clinical Research Network initiative that is described in detail below. This network will be evaluating promising new therapies for diabetic eye diseases and will use standardized data reporting approaches that are being emphasized in the NIH roadmap initiative on clinical research. This network approach may also serve as an example for the development of clinical research networks to evaluate promising new therapies and preventive strategies for other ocular diseases.
NEI New Initiatives
Diabetic Retinopathy Clinical Research Network. The NEI will further expand its initiative to support core centers to plan, implement, and conduct clinical trials of the treatment of diabetic retinopathy, diabetic macular edema, and associated conditions. Establishment of the clinical research network of core centers and participating clinics will help satisfy the need to evaluate promising new approaches to treat diabetes induced retinal disorders and to investigate other approaches as they become available. More than 70 clinical centers with the capability to participate in the clinical trials network have been identified. The NEI, in conjunction with the clinical trials network, has developed innovative techniques for wireless, real-time, hand-held, distributed data entry that will be used in each clinical center. In addition, this collaboration has developed a novel automated device and methodology for measuring visual acuity. This network approach would provide a framework for rapid initiation of important studies, efficient use of pooled clinical expertise in idea generation and protocol development, and efficient use of central resources for data management, quality control, and endpoint evaluation.
National Ophthalmic Genotyping Diagnostic and Research Network. The rapid progress in areas of gene discovery and bioinformatics has created the need for enhanced cooperation and coordination among groups that provide genetic diagnostic information to the clinician and patient, store and provide DNA specimens to researchers, and maintain data banks of genotype-phenotype information. Such groups are underrepresented in the area of human ocular disease. The purpose of this initiative is to explore the establishment of a national central registry and molecular database of securely coded information from a large number of people with ocular diseases caused by genetic mutations. Information will be provided through a network of cooperating groups who provide genetic and diagnostic services to patients and clinicians. Such a registry and database will be of great value in advancing research in this area.
Innovations in Management and Administration
NEI Strategic Planning. The NEI recently published and released its National Plan for Eye and Vision Research, the first strategic plan produced through the new, two-phase planning process. This ongoing planning process involves the assessment of important areas of progress in eye and vision research and the development of new goals and objectives that address outstanding needs and opportunities for additional progress. Workshops, conferences, or symposia in critical or emerging areas of science are being conducted during the second phase of the planning process to explore how new findings and technologies might be applied to diseases of the eye and disorders of vision. The National Plan and information on planning workshops and conferences can be accessed through the NEI website at: http://www.nei.nih.gov/strategicplanning.
Budget Policy
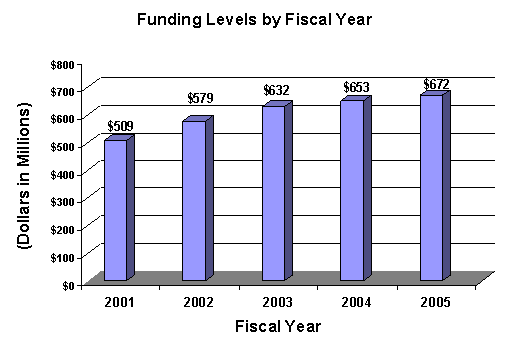
The Fiscal Year 2005 budget request for the NEI is $671,578,000 an increase of $18,840,000 and 2.9 percent over the FY 2004 Final Conference Level. Also included in the FY 2005 request, is NEI's support for the trans-NIH Roadmap initiatives, estimated at 0.63% of the FY 2005 budget request. This Roadmap funding is distributed through the mechanisms of support, consistent with the anticipated funding for the Roadmap initiatives. A full description of this trans-NIH program may be found in the NIH Overview.
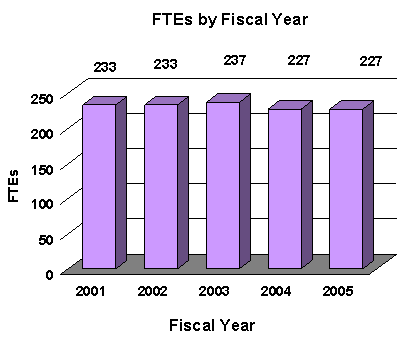
A five year history of FTEs and Funding Levels for NEI are shown in the graphs below. Note that the Fiscal Year 2001 FTE figure is not comparable to the figures in the succeeding years due to NIH's consolidation of its Human Resources function in FY 2003.
|
|
NIH's highest priority is the funding of medical research through research project grants (RPGs). Support for RPGs allows NIH to sustain the scientific momentum of investigator-initiated research while providing new research opportunities. The FY 2005 NIH request provides for an aggregate 1.3 percent increase in average cost for Research Project Grants, consistent with the Gross Domestic Product Deflator. The NEI is providing an average cost increase of 1.9 percent for direct recurring costs in noncompeting continuation awards. Competing RPGs are based on an average cost increase of 1 percent.
Advancement in medical research is dependent on maintaining the supply of new investigators with new ideas. In the Fiscal Year 2005 request, NEI will support 301 pre- and postdoctoral trainees in full-time training positions. Stipend levels for pre-doctoral and post-doctoral recipients supported through the Ruth L. Kirschstein National Research Service Awards will remain at FY 2004 levels.
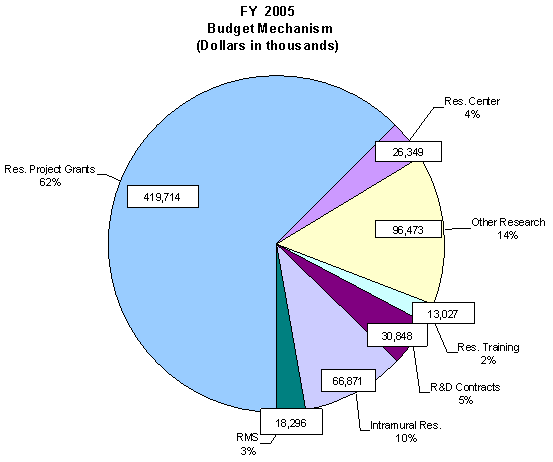
The Fiscal Year 2005 request includes funding for 41 research centers, 190 other research grants, including 59 clinical career awards, and 99 R&D contracts. Intramural Research and Research Management and Support receive increases to support increased pay and estimated inflationary increases in FY 2005. Within the Intramural Research program, NEI scientists will participate in a coordinated NIH intramural obesity research program and provide ophthalmic consultation services for NIH obesity-related clinical protocols.
The mechanism distribution by dollars and percent change are displayed below:
|
|
1 Sperduto R, Seigel D, Roberts J, Rowland M: Prevalence of myopia in the United States. Arch Ophthalmol 101(3):405-407, 1983.
2 Prevent Blindness America. Vision Problems in the U.S.: Prevalence of Adult Vision Impairment and Age-Related Eye Diseases In America. 36 pp. (2002)
3 Hillis A, et al: The evolving concept of amblyopia: a challenge to epidemiologists. Am J Epidemiol 118(2):192-205, 1983.
4 Preslan M, Novak A: Baltimore Vision Screening Project. Ophthalmology 103(1):105-109, 1996.
5 Tielsch J, Sommer A, Will K, Katz J, Royall R: Blindness and visual impairment in an American Urban population, the Baltimore Eye Survey. Archives of Ophthalmology 108:286-290, 1990.