 |
|
 |

Colorectal Cancer Drugs Require Careful Patient Selection
Patients with advanced colorectal cancer who have mutant forms of the gene KRAS in their tumors should not receive chemotherapy plus cetuximab 1 (Erbitux), because they are unlikely to benefit from the treatment and should be spared the side effects and cost, researchers said at the recent American Society of Clinical Oncology (ASCO) annual meeting in Chicago.
Based on a growing body of evidence, including findings presented at the meeting, several experts predicted that it will become standard practice to test all colorectal tumors for mutations in the KRAS gene before starting patients with advanced disease on therapies involving cetuximab and a similar drug, panitumumab 2 (Vectibix).
"I believe it is now warranted to test all patients being considered for these agents," said Dr. Gail Eckhardt of the University of Colorado Denver, who was not involved in the research and discussed the findings at ASCO. "Patients with KRAS mutations should not receive cetuximab or panitumumab in [certain] settings."
Read more 3 


Gemcitabine after Pancreatic Cancer Surgery Improves Survival
Patients who received the chemotherapy drug gemcitabine 4 after surgery for pancreatic cancer lived 2 months longer than patients who had surgery alone, according to the final results 5 of a large, randomized clinical trial presented at the ASCO annual meeting last week.
Less than 20 percent of patients with pancreatic cancer are candidates for surgery, because the disease is often detected in the late stages. Gemcitabine has been a standard treatment for patients with advanced (and inoperable) pancreatic cancer for a decade. The new findings support use of the drug in the adjuvant setting.
Read more 6 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Colorectal Cancer Drugs Require Careful Patient Selection
Patients with advanced colorectal cancer who have mutant forms of the gene KRAS in their tumors should not receive chemotherapy plus cetuximab 1 (Erbitux), because they are unlikely to benefit from the treatment and should be spared the side effects and cost, researchers said at the recent American Society of Clinical Oncology (ASCO) annual meeting in Chicago.
Based on a growing body of evidence, including findings presented at the meeting, several experts predicted that it will become standard practice to test all colorectal tumors for mutations in the KRAS gene before starting patients with advanced disease on therapies involving cetuximab and a similar drug, panitumumab 2 (Vectibix).
"I believe it is now warranted to test all patients being considered for these agents," said Dr. Gail Eckhardt of the University of Colorado Denver, who was not involved in the research and discussed the findings at ASCO. "Patients with KRAS mutations should not receive cetuximab or panitumumab in [certain] settings."
These drugs are designed to block the activity of the epidermal growth factor receptor (EGFR) protein, which is often overactive in colorectal cancer.
An estimated 30 to 40 percent of colorectal tumors carry KRAS mutations, and commercial tests are available. Screening could proceed based on the breast cancer model, where women undergo testing for genetic characteristics of their tumors prior to treatment with trastuzumab 7 (Herceptin).
"KRAS is the first molecular marker for targeted therapy in combination with standard chemotherapy as a first-line treatment for metastatic colorectal cancer," said Dr. Eric Van Cutsem of Gasthuisberg University Hospital in Leuven, Belgium, at the meeting. "If we know in advance that a patient has a KRAS mutation, then we know we don't have to treat the patient [with these agents]."
He presented new results 8 from the CRYSTAL trial, which last year 9 showed that some patients with metastatic disease benefited from cetuximab plus chemotherapy with respect to progression-free survival. But not all patients benefited and given growing interest in the KRAS gene, the researchers went back and looked at tumor tissue from 587 of the nearly 1,200 patients in the trial.
The results were striking: Only patients with normal KRAS genes benefited. Perhaps most important, findings from other studies, including the OPUS 10 and EVEREST 11 trials, support the findings from the CRYSTAL trial. Retrospective analyses of KRAS gene status and treatment outcomes have now been performed on 1,200 patients with advanced colorectal cancer from separate randomized trials.
"We now have substantial evidence that mutations in KRAS are a negative predictive marker for the use of cetuximab with chemotherapy and for panitumumab as a single agent based on results from a variety of trials," said Dr. Margaret Mooney of NCI's Cancer Therapy Evaluation Program 12, who was not involved in the research.
Before the meeting, European regulators approved cetuximab plus chemotherapy as a first-line treatment for colorectal cancer in patients with normal KRAS. Panitumumab is approved in Europe for treating advanced colorectal cancer, but also only in patients with the normal KRAS gene.
Prospective studies are now needed to validate the marker. Trials such as CRYSTAL were not designed to answer questions about cetuximab and KRAS, and the researchers do not have tumor tissue from all patients. These patients could provide useful information in developing new therapies.
Dr. Eckhardt stressed the importance of communicating to patients with KRAS mutations that current chemotherapy regimens are effective. "Hopefully," she added, this is "only the beginning of the era of individualized therapy for patients with colon cancer."
—Edward R. Winstead
|
 |
 |
Gemcitabine after Pancreatic Cancer Surgery Improves Survival
Patients who received the chemotherapy drug gemcitabine 4 after surgery for pancreatic cancer lived 2 months longer than patients who had surgery alone, according to the final results 5 of a large, randomized clinical trial presented at the ASCO annual meeting last week.
Less than 20 percent of patients with pancreatic cancer are candidates for surgery, because the disease is often detected in the late stages. Gemcitabine has been a standard treatment for patients with advanced (and inoperable) pancreatic cancer for a decade. The new findings support use of the drug in the adjuvant setting.
"We have shown that this treatment more than doubles the overall survival 5 years after treatment," said Dr. Helmut Oettle of the Charité School of Medicine in Berlin, Germany, who presented the results.
The study included 368 patients who underwent surgery followed by 6 months of adjuvant gemcitabine treatment or surgery alone. In the gemcitabine group, 21 percent were alive at 5 years compared with 9 percent in the control group. Median survival in the gemcitabine group was 22.8 months compared with 20.2 months in the control group.
Preliminary results from the trial were reported at ASCO in 2005 and showed that post-surgery gemcitabine could delay a recurrence of the disease. These findings led to an increase in the use of the drug in the United States and Europe, according to the researchers.
"We can now say that giving this agent after surgery to patients with early stage disease will improve a patient's survival," commented Dr. Nicholas Petrelli of the Helen F. Graham Cancer Center at the meeting. "We couldn't say that before."
Cetuximab Plus Chemotherapy Extends Survival for Advanced Lung Cancer
Patients with advanced non-small-cell lung cancer (NSCLC) who received cetuximab 1 (Erbitux) plus chemotherapy lived on average 5 weeks longer than patients who received chemotherapy alone, according to results 13 reported at the ASCO annual meeting.
In the phase III FLEX trial, 1,125 patients with all types of NSCLC were randomly assigned to receive standard platinum-based chemotherapy alone or chemotherapy plus cetuximab. Nearly all of the patients had stage IV disease. Overall survival was higher for those who received cetuximab plus chemotherapy (11.3 months) compared with those who received chemotherapy alone (10.1 months).
The benefit of the combination therapy was seen in patients with all histological subtypes of NSCLC, including adenocarcinoma and squamous cell carcinoma, the two most common subtypes. The main side effect was an acne-like skin rash that could be managed.
"Patients with advanced NSCLC have limited treatment options and life expectancy is short, so the survival increase shown in this study is an important step for these patients," noted Dr. Robert Pirker, an associate professor of medicine at the Medical University of Vienna in Austria and the study's lead author.
The only other final-stage randomized trial to show a survival benefit in lung cancer was a 2005 study 14 of bevacizumab 15 (Avastin) plus chemotherapy. Unlike the current study, that trial did not include patients with squamous cell carcinoma.
Dr. Thomas Lynch of Massachusetts General Hospital, who commented on the findings at ASCO, said the study was well done and produced "a clinically meaningful benefit for a large population."
Zoledronic Acid Improves Early Breast Cancer Treatment
The addition of zoledronic acid 16 (Zometa) to adjuvant endocrine therapy in premenopausal women with early stage breast cancer significantly improves clinical outcomes beyond those achieved with endocrine therapy alone, researchers reported at the ASCO annual meeting. The results 17 are from a phase III randomized trial of 1,800 women conducted by the Austrian Breast and Colorectal Cancer Study Group.
Zoledronic acid, part of a class of drugs known as bisphosphonates, is already used to treat bone metastases 18, and this trial was conducted based on data from preclinical and early phase trials indicating that the drug can also shrink tumors and block metastatic activity. The results bear this out, said principal investigator Dr. Michael Gnant from the Medical University of Vienna.
Overall, the trial showed no difference in disease-free survival between women treated with tamoxifen or anastrozole. But the addition of zoledronic acid to either therapy decreased the risk of a disease-free survival event by 36 percent compared with hormone therapy alone, Dr. Gnant said. At a median follow-up of 60 months, overall disease-free survival was 92.4% and overall survival was 97.7%.
Women in the trial - all of whom were premenopausal with stage I or II breast cancer that was responsive to endocrine therapy - were treated with surgery and, if needed, radiation therapy. They also received the drug goserelin to temporarily suppress the function of the ovaries. Each was randomly assigned to one of four adjuvant therapy arms: tamoxifen 19 alone, the aromatase inhibitor anastrozole 20 alone, or either drug plus zoledronic acid. Treatment lasted 3 years.
The drug was also well tolerated, with no indication of increased risk of liver problems or damage to the jaw bone - two side effects which have been associated with higher doses of bisphosphonate drugs.
Acupuncture Tested in Cancer Patients after Neck Surgery
For patients with head and neck cancers who undergo a surgical procedure known as neck dissection, acupuncture may help reduce pain and improve functioning afterward, according to preliminary findings 21 from a randomized trial presented as a poster at ASCO.
"Acupuncture appears to be a promising treatment for these patients, and more research is warranted," said Dr. David Pfister of Memorial Sloan-Kettering Cancer Center, who discussed the results at ASCO. Conventional treatments have limited success in helping patients following neck dissections, he noted, and "there is plenty of room for improvement."
The study included 34 patients who received acupuncture and 36 who received standard care with pain medication and physical therapy. In the experimental group, four sessions of acupuncture were administered over the course of a month. A standard set of acupuncture points was used for each patient, and more specialized points were selected depending on the pain for each individual.
Thirty-nine percent of the patients who received acupuncture had significant improvements in pain and function, compared with only 7 percent who experienced improvement in the standard care group, according to assessments using a measure called the Constant-Murley scale. Some patients in the acupuncture group had significant relief of dry mouth, while no relief was observed in the patients receiving standard care.
Future studies may include a group that receives "sham" acupuncture to control for the placebo effect.
Protein Biomarkers Point to Early Stage Pancreatic Cancer
Researchers have discovered proteins in blood that reliably indicate early stage pancreatic cancer, according to a report June 10 in PLoS Medicine 22. Although more research is needed before an actual diagnostic test could be developed, the study's lead author, Dr. Samir Hanash from the Fred Hutchinson Cancer Research Center, said the study represents a "breakthrough in the application of advanced proteomic technologies and mouse models to cancer-biomarker discovery."
The mouse model used in the study was genetically engineered to mimic the course of pancreatic cancer in humans, from development of precancerous lesions through advanced disease. Using proteomic technologies, the researchers identified a panel of five proteins - LCN2, REG1A, REG3, TIMP1, and IGFBP4 - consistently found in mice with precancerous growths called pancreatic intraepithelial neoplasia, but not in mice with full-blown cancer or healthy control mice.
To validate the five-protein panel, they tested it against blood samples from 13 people in an unrelated cancer prevention study who developed pancreatic cancer within a year of donating the sample. The researchers were "blinded" to which samples came from cancer patients and controls. The five-protein panel consistently identified samples from the patients who developed cancer, and when it was combined with another protein marker, CA19.9, which is elevated in up to 80 percent of newly diagnosed pancreatic cancer patients, the test was even more accurate.
The next steps include studies to validate the biomarker panel's performance in distinguishing between pancreatitis (inflammation of the pancreas) and pancreatic cancer - under the auspices of NCI's Early Detection Research Network 23 (which also partly funded this current study, along with NCI's Mouse Models of Human Cancers Consortium 24) - and continued studies to assess its value in early detection among those at high risk of pancreatic cancer.
Initial Costs of Cancer Treatment on the Rise
In the first study to examine trends in the costs of specific components of initial cancer care in the United States, investigators found that Medicare payments for initial treatment rose significantly for breast, colorectal, and lung cancer patients but dropped slightly for prostate cancer patients between 1991 and 2002. These results, published online June 10 in the Journal of the National Cancer Institute, highlight the financial challenges to Medicare 25 posed by the rising number of cancer patients in the United States as the population ages, the study authors said.
The investigators used the linked Surveillance, Epidemiology, and End Results-Medicare 26 data from 306,709 newly diagnosed breast, colorectal, lung, and prostate cancer patients age 65 or older. Specific services evaluated included cancer-related surgery, chemotherapy, radiation therapy, and hospitalizations during the initial treatment period (defined as from 2 months prior to 12 months following diagnosis). The investigators assessed the proportion of patients who were hospitalized, received cancer-related surgery, chemotherapy, and radiation therapy, as well as the average cost per patient for those services.
After adjusting for inflation, the investigators found that between 1991 and 2002 the average cost per patient rose $7,139, $5,345, and $4,189 for lung, colorectal, and
breast cancer, respectively. Prostate cancer costs decreased by $196, due to a decline in the use of radical prostatectomy. The most significant increases were due to the percentage of breast, lung, and colorectal cancer patients receiving chemotherapy and the average cost per patient for chemotherapy. Over this time period the proportion of breast and prostate cancer patients receiving radiation therapy increased, as did the cost of radiation therapy. However, hospitalizations during the initial treatment period accounted for the greatest portion of total Medicare payments.
The study ended before the introduction of new, more expensive
chemotherapies and targeted
therapies such as erlotinib 27 and bevacizumab 15, noted the authors. "Evaluation of the impact of these
new agents on total Medicare expenditures for initial cancer care will be
a priority for future research," said the lead author, Dr. Joan Warren
from NCI's Division of Cancer Control and Population Sciences 28.
|
 |
 |

Dr. Niederhuber's Remarks at the ASCO Opening Session
The following is an abridged version of the prepared remarks delivered by NCI Director Dr. John Niederhuber on May 31 during the opening session of the 2008 ASCO Annual Meeting in Chicago, IL. This text represents the basis of his remarks and may have been modified slightly during delivery.
Good morning and thank you for asking me to appear before you today. … We are extremely fortunate to have such outstanding leaders of [ASCO] - individuals who so clearly understand the times we live in and the challenges and opportunities that lie before us - as we lead a transformation of science and medicine in the post-human-genome era. Among our many challenges, there is none, I believe, greater than the necessity that we more rapidly translate our discoveries, our new interventions, to patients.
As oncologists, as cancer physicians, we become intimate with the fears of our patients. Sitting at the bedside, gazing into the frightened, tearful eyes of far too many men, women, and children just learning they have cancer, we know that statistics are never simply numbers. They are very real individuals, each in his or her own way grasping to cope with news that, as a young physician being treated for Hodgkin disease recently reminded me, "is a life-changing event."
Recently, cancer has deeply touched us. Last year, at this podium, we honored a lifetime of dedicated service and leadership by our great friend and revered colleague Marty Abeloff. This year, we are all diminished by his absence. His loss, although months past, is still fresh, still palpable, and still painful to his colleagues and friends.
We also remember - I, especially - Judah Folkman, a legendary surgeon, educator, and researcher, whose skill, vision, and compassion we dearly miss and shall never forget.
Cancer intrudes into our national consciousness, as well. Just a few weeks ago, the Honorable Edward Kennedy chaired a special hearing of the Senate Health, Education, Labor, and Pensions Committee to consider ways our country could invest in and reinvigorate its collective efforts against cancer. Today, Senator Kennedy is courageously negotiating the treacherous path of the very disease he has worked so hard, for so many years, to fight. It is impossible to escape the irony that one of the architects of the landmark National Cancer Act of 1971 now turns his battle inward.
I daresay none of us has escaped cancer's scars, whether we wear those marks personally or symbolically, on behalf of a loved one.
As a nation, I believe we must confront the fact that a rapidly aging population and its cancer burden will weigh heavily, not only on health care, but on the workforce and our economy. As cancer researchers and cancer physicians, we face an inescapable question: Can we sustain our momentum and continue to lessen the all too devastating impact of cancer?
I believe that we are all here in Chicago this week, thousands strong, to resoundingly answer: "Yes."
We are here to listen and learn and share and discuss - and even debate - the promise of science and the assurance that we will not rest until that promise is delivered to all of our cancer patients, whether they seek treatment in a world-renowned university cancer center or in a rural community hospital. And we must resolve to consign to history the inequalities of cancer treatment that arise from age, frailty, race, ethnicity, poverty, and language.
As director of the National Cancer Institute - your National Cancer Institute - I stand here this morning to recommit NCI to make every effort toward the healthy tomorrows we all envision. I extend this promise fully aware of the challenges [presented by 4 straight years of funding that have been consistently below the rate of medical inflation].
I also know that our cancer patients don't want to hear explanations about flat budgets. They want prevention options. They want effective, novel therapies. NCI has an obligation, to our patients and to our country, to set the agenda for and to facilitate cancer research in the decades ahead. I believe, as well, that NCI is uniquely poised to bring together industry, academia, and government in the search for solutions none could provide by itself.
We come together during a time of unparalleled discovery, of rapidly mounting information about the alterations of the code of life that become aberrant and lead to cancer. Consider, if you will, The Cancer Genome Atlas 29, often called TCGA for short, to denote the four DNA bases. Co-sponsored by NCI and our sister NIH institute, the National Human Genome Research Institute, this 3-year pilot project 30 is an effort to test the feasibility of large-scale characterization and sequencing of patients' tumors. We have sequencing centers up and running, along with tissue characterization centers making sure samples archived for sequencing (up to 500 samples of both tumor and matched normal tissue) are of sufficient quality.
The first cancers being studied in this pilot program are lung, ovarian, and glioblastoma multiforme. In part, because high-quality tumor tissue has been available, glioblastoma has led the way in this effort. To date, more than 234 tumors have undergone comprehensive characterization and 1,300 genes have been sequenced, and some fascinating associations have been revealed - specifically, two genes that are highly associated with glioblastoma: NF1 and Erbb2. Neither of these genes was previously known to have an association with this disease. In addition, previously unknown changes in the EGF receptor and the p53 tumor suppressor gene are now known to be part of glioblastoma's course. Based on this new, very preliminary analysis, at least four general subtypes of glioblastoma are emerging.
Tumor sequencing of this sort, along with the rapidly advancing field of whole-genome scanning, are, without a doubt, at the forefront of cancer science. Yet, for now, this is just information: a collection of T's, C's, G's, and A's. Our challenge is to convert this powerful information into knowledge of how gene expression is transcriptionally regulated; how that expression is translated into proteins affecting signal pathways; and how these changes eventually alter tissue function and disease phenotype. These determinations will not be easy.
Ultimately, the power of the genome will be to push forward the development of new strategies in prevention, new opportunities for early detection, essential biomarkers of disease, and, of course, novel drugs and new treatment approaches. We must be focused on enabling translation.
This need was made clear in a report in 2007 of NCI's Translational Research Working Group 31, which spent 2 years conducting a detailed analysis of how to realize the power of a bench-to-bedside approach. The report called on NCI to tailor both new and existing programs to facilitate early translational research progress. NCI is working hard to answer the report's call in five key ways.
The first area is clinical research. We are fortunate to have, on the NIH campus in Bethesda, the world's largest hospital principally devoted to clinical studies. It is the home to a dedicated cadre of NCI intramural science programs and scientists. But we have also come to believe that, in order to facilitate the best translational efforts, this outstanding facility should become a resource for the extramural community. For the first time, we have a trial open with an extramural co-principal investigator, and there are several more such trials pending.
Additionally, we are reaching out to both the new Walter Reed National Military Medical Center, which is due to begin construction across the street from NIH this summer, and Suburban Hospital, which is across the street on the other side of campus. These two facilities have the potential to give us an opportunity to work on prevention and to see more early stage patients.
Our second goal is drug development. NCI often works with orphan opportunities: small molecules and biologics that are not being developed by industry because they lack a significant market share or they are too risky to pursue. We must enable that work. We see drug development as a platform that encompasses a new chemical biology consortium and extends NCI's Developmental Therapeutics Program 32. We have a duty, I believe, to develop new agents and to carry them forward to first-in-human studies.
Drug development will make our third goal essential: Reaching out to work with both the Food and Drug Administration and the Centers for Medicare and Medicaid Services, connecting regulators and those responsible for reimbursement. Just one example is our Interagency Oncology Drug Task Force 33 that, among other functions, sponsors NCI-FDA fellowships to train young scientists in research and in research-related review, policies, and regulations.
Public-private partnerships will also be increasingly necessary. As we move to a more personalized era of oncology, it is clear that we will require multiple agents to target multiple pathways in the same patient. Facilitating that future will challenge how we think of competition, intellectual property, and even the language of contracts. I believe NCI must step into those areas and become the facilitator between the public, private, and academic sectors. I believe it is a fair question to ask: If not NCI, then who will lead this absolutely vital effort?
Fifth and finally, if NCI is to appropriately facilitate translation, we must make every attempt to provide stable funding for some of the most important programs at the heart of translational research. Specifically, I am referring to clinical trials, the Specialized Programs of Research Excellence 34 (SPOREs), the Community Clinical Oncology Program 35 (CCOPs), and the Cooperative Groups 36.
Although it is entirely possible that NCI's below-inflation funding may continue for several more years, we will do - and must do - all we can to keep these crucial programs vigorous.
Overarching all that we do, NCI has a duty to make every effort to reduce the disparities that affect cancer prevention and treatment. I am convinced that access to our latest science will be the greatest determinant of cancer mortality in the years to come.
To address this we are now beginning the second year of our NCI Community Cancer Centers pilot program 37, a research effort to study how we can best bring state-of-the-art, multi-specialty care, and earliest-phase clinical research - phase I and phase II studies - to patients in the communities where they live. It gives me great pride to hear the 16 community hospital [pilot] sites report on their enthusiastic efforts, and how much they have been able to leverage the modest funding provided by NCI. It is also extremely gratifying to know that these pilot sites, seven of which have links to the CCOP program, are participating in ASCO's Quality Oncology Practice Initiative.
All of NCI's efforts - and certainly all of your efforts as oncologists - are about individuals with cancer. We have made many, many advances, and today we are witnessing perhaps the most exciting era in science's history.
I would like to conclude this morning with the story of a patient. It is a story not about a common occurrence or even one that may be replicated. It is a story about the power and the importance of your National Cancer Institute. It is a story of NCI's work in drug development and of facilitating early translational research.
When our patient arrived at the NIH Clinical Center, she couldn't even make a fist. Her hands, wrists, elbows, and knees could scarcely bend. Her skin was cracking, and she suffered the pain of many open skin lesions. Her face was swollen and disfigured. A once-vibrant woman in her late twenties, she was now severely anemic, wheelchair bound, and wrapped in blankets to preserve the body heat her skin could no longer retain. Over 2 years, as she suffered the disabling and constantly progressive manifestations of cutaneous T-cell lymphoma, the nights spent in the hospital had come to greatly outnumber those spent at home.
She was in hospice care and lacked the strength to be with her two small children. She came to the Clinical Center virtually out of treatment options, and once there, an initial short list of experimental treatments all failed.
Having apparently run out of hope, our patient's story came to the attention of Dr. Martin Gutierrez 38, a staff clinician with NCI's Medical Oncology Branch 39. Dr. Gutierrez, who has spent his career working with T-cell lymphoma patients, suggested trying a new drug being developed through NCI's well-known RAID Program 40. The Rapid Access to Intervention Development Program, as you are well aware, exists to speed the translation of novel anticancer therapies from our laboratories into first-in-human studies.
The drug utilized by Dr. Gutierrez, fenretinide, was one that was developed but never marketed by a pharmaceutical company as a chemopreventive agent. It was brought to the RAID program by an extramural investigator from Los Angeles, who wanted to reformulate it as a potential chemotherapeutic drug, based on observations he had made in laboratory models.
In this case, the drug paid off, and paid off dramatically. Within the first few doses, Dr. Gutierrez began to see remarkable improvement. Within 7 months, the patient's symptoms were completely gone. Today, almost 2 years after her arrival at the Clinical Center, the patient's tests show no evidence of disease.
To me this story - not a report of a large clinical trial, but involving just one patient - illustrates many aspects of the role of NCI. It was not the most common cancer problem and not the most common potential therapeutic compound. Instead, it is a great example of the partnerships between the extramural community and NCI in the development of novel agents. It is, I believe, an example of the increasing need for the development of new therapies that are not attracting the prime interest of the private sector. It is about knowing the science and using that knowledge to drive drug discovery; about using genetics to drive high-throughput screening; about the power of a large workforce of chemists to address formulation; and about scientific opportunity, without the consideration of shareholder profit.
We are all looking to the day when stories like this are commonplace, when we make cancer a more chronic, manageable condition.
We have many miles and, yes, a few mountains ahead of us on [our] path. My commitment to you is that your National Cancer Institute will walk beside you, helping you negotiate every step.
May our days here in Chicago renew our spirits, renew our commitment, and recharge us for the walk ahead. Thank you for all you do and for your support, and thank you for being here this morning.
|
 |
 |

Ovarian Cancer Study Could Speed Early Detection
Can a simple blood
test detect early signs
of ovarian cancer? This question has been asked repeatedly over the last decade, and an answer may finally come this summer.
In a closely watched study, five research groups are validating their most promising ovarian cancer markers using high-quality blood samples from the Prostate, Lung, Colorectal and Ovarian 41 (PLCO) cancer screening trial, including some prediagnostic samples from women who developed ovarian cancer during the trial.
 The validation study, coordinated by NCI's Early Detection Research Network 23 (EDRN), could show whether panels of markers can detect ovarian cancer in blood collected 6 months or more before cancer was discovered. If markers do predict ovarian cancer, the question then becomes: How long before a clinical diagnosis are the markers informative?
The validation study, coordinated by NCI's Early Detection Research Network 23 (EDRN), could show whether panels of markers can detect ovarian cancer in blood collected 6 months or more before cancer was discovered. If markers do predict ovarian cancer, the question then becomes: How long before a clinical diagnosis are the markers informative?
Earlier is always better, and for this disease in particular. Ovarian cancer has been called a "silent killer" because the vast majority of cases go undetected until the later stages, when women have few treatment options. The hope now is that a blood test for early detection is within reach.
"Everybody has a real sense of urgency now," said Dr. Daniel Cramer of Brigham and Women's Hospital, who leads one group. "We've been saying for years that we'll get a marker for ovarian cancer, but it hasn't happened yet. This is the first good test to see if we can come up with a panel of markers for ovarian cancer from PLCO samples."
The PLCO is one of several large, randomized studies that have been making limited samples available for biomarker research based on proposals from investigators. These studies have rare prediagnostic samples that were collected before anyone knew that a person had a particular cancer. Such samples are considered "more pure" and may more accurately reflect the biology of ovarian cancer than other specimens.
The vast majority of prediagnostic blood samples for ovarian cancer are collected from women just prior to surgery for a suspected ovarian tumor. But impending surgery and anesthesia, for example, might alter at least some potential markers in women with the cancer but perhaps not in a comparison group.
 |
 |
Missed a Highlight? |
 |
|
The NCI Cancer Bulletin Archive 42 allows you to search every issue of this online publication since January 2004. That's more than 180 issues' worth of articles on a variety of cancer research topics and updates.
|
|
The PLCO biorepository has serial blood samples from 150,000 people collected at 10 sites across the country, making it a veritable gold mine for biomarker research. Because samples were collected and stored before a diagnosis was known, the researchers minimized the chances of inadvertently introducing a bias or systematic difference between the cancer and comparison groups.
Similarly, in the validation study, great care was taken to eliminate factors that, in the end, could mislead investigators.
"If the biomarkers do not detect ovarian cancer in these samples, it will be because of the biology of ovarian cancer and not because of how the study was designed or implemented," said Dr. Christine D. Berg of NCI's Division of Cancer Prevention 43 and the leader of PLCO.
The validation study is being managed by Dr. Christos Patriotis of NCI's Cancer Biomarkers Research Group 44 with Dr. Sudhir Srivastava, who heads the EDRN program.
The study could yield a variety of markers, including some that improve the sensitivity of the protein CA-125 test. This marker is routinely evaluated when diagnosing ovarian cancer, but it cannot be used alone because only some women with ovarian cancer develop elevated levels, and levels can be elevated for reasons other than cancer.
"Many potential biomarkers have been proposed to be added to CA-125 for the early detection of ovarian cancer, and the PLCO samples will help us select the most promising of those markers," said Dr. Robert Bast, Jr., of the University of Texas M.D. Anderson Cancer Center, who discovered CA-125 and is an investigator in the study.
Beyond early detection and diagnosis, markers are needed to assess risk and guide difficult clinical decisions. For instance, some women with genetic risk factors for breast and ovarian cancers have their ovaries and fallopian tubes removed as preventive measures after childbearing years.
The validation study could yield markers that identify women without the risk genes who nonetheless have a high risk of the disease within 5 to 10 years. Other markers could identify women who have a family history of ovarian cancer but are at such low risk that they could safely avoid preventive surgeries, at least in the short term.
Before any of these markers can be used in the clinic, they would need to be tested prospectively, and the challenges of early detection are substantial.
"It may be helpful to think about what we're trying to do," said Dr. Nicole Urban of Fred Hutchinson Cancer Research Center, another lead investigator. "With a screening program, a woman comes in and you get a blood sample and try to predict if she's going to be diagnosed with cancer. You're trying to find cancer that would kill the woman, but you're trying to find it early enough that you can cure it. And this is tough."
Dr. Urban is nonetheless optimistic that screening for ovarian cancer using markers will ultimately succeed. She predicts that serial blood samples from randomized trials may be required to discover the markers and track their changes over time.
"There is no single marker that will be sensitive enough to distinguish all the ovarian cancer patients from healthy women," said Dr. Gil Mor of Yale University, who leads the fourth group. His team has developed panels with two types of markers - proteins produced by ovarian tumors and proteins produced by the body in response to very early changes associated with ovarian cancer.
All of the groups have taken essentially similar strategies, noted Dr. Anna Lokshin at the University of Pittsburgh Cancer Institute, the principal investigator for the fifth study group. "We are all now waiting for the results." The data are being analyzed, and answers could come within months.
"A lot of people around the country are holding their breath to see the results," said Dr. David Ransohoff, a cancer epidemiologist
at the University of North Carolina
at Chapel Hill, who consulted on
the project.
"These are some of the best groups in the country; they are investigating an important question; and the specimens are among the best available to answer it," he continued. "If the results are positive in these samples, it will be enormously good news for the field."
—Edward R. Winstead
|
 |
 |

FDA Conducting Safety Review of TNF Blockers, Adds Warning to Becaplermin
The Food and Drug Administration (FDA) is investigating the possible association between drugs known as tumor necrosis factor 45 (TNF) blockers and the development of several cancers, primarily lymphomas. The drugs - sold in the United States as Remicade, Enbrel, Humira, and Cimzia - are used to treat children and young adults for juvenile idiopathic arthritis and Crohn disease 46, as well as other diseases, FDA explained in an "early communication" about the investigation.
The review 47 follows 30 reports of cancer in children and young adults (age 18 or under) submitted via the agency's Adverse Event Reporting System since 1998, when the first TNF blocker was approved. Of the cancers reported, FDA said, approximately half were lymphomas, including Hodgkin and non-Hodgkin lymphoma.
"While cancers are known to occur in children and young adults, the reports of these events in children and young adults receiving TNF blockers are of concern and deserve further investigation," FDA said. "Long-term studies are necessary to provide definitive answers about whether TNF blockers increase the occurrence of cancers in children because cancers may take a long time to develop and may not be detected in short-term studies."
Last week, FDA also announced that it was adding a boxed warning 48 to the prescribing information of becaplermin (Regranex), a topical cream indicated for the treatment of leg and foot ulcers that are not healing in diabetic patients, about an increased risk of cancer mortality associated with use of three or more tubes of the product.
The warning, FDA explained, is based on the results of two studies that have shown an increased cancer mortality risk among those who have used three or more tubes of becaplermin. In one retrospective study, such use of becaplermin, a recombinant human platelet-derived growth factor, was associated with a five-fold increased risk of cancer mortality.
The studies did not identify any specific cancers for which the product might increase mortality risk.
|
 |
 |
 Wlodawer Awarded Heyrovsky Medal
Wlodawer Awarded Heyrovsky Medal
Last month, Dr. Alexander Wlodawer, chief of the Macromolecular Crystallography Laboratory 49 in NCI's Center for Cancer Research, received the Jaroslav Heyrovsky Honorary Medal for Merit in Chemical Sciences. The medal,
established in 1965, is awarded by
the President of the Academy of Sciences of the Czech Republic and recognizes outstanding scientific results in the field of chemistry. Dr. Wlodawer was recognized for his important contribution to the studies of protein structure and also for his advisory role in the revitalization of the Institute of Organic Chemistry and Biochemistry in Prague.
 Francis Collins Leaves NHGRI
Francis Collins Leaves NHGRI
Dr. Francis Collins recently announced that he will step down as director of the National Human Genome Research Institute (NHGRI) after 15 years leading NIH efforts to map the human genome. Dr. Collins will pursue new professional opportunities and write a book about the use of genetic research to personalize medical prevention and treatment.
NIH Director Dr. Elias Zerhouni referred to Dr. Collins as a "trailblazer in the scientific community." Dr. John Niederhuber, director of NCI, also praised Collins' leadership noting NHGRI's partnership with NCI during the pilot phase of their joint venture, The Cancer Genome Atlas. This landmark project to understand the molecular basis of cancer through genome characterization "is an example of the kind of groundbreaking research two institutes can create, when their focus is on nothing less than the best science for the benefit of patients," Dr. Niederhuber commented. "What Dr. Collins has contributed to these pioneering efforts will one day transform cancer medicine."
Free Telephone Workshop for Cancer Survivors
CancerCare, in collaboration with NCI, the Lance Armstrong Foundation, Intercultural Cancer Council, Living Beyond Breast Cancer, and National Coalition for Cancer Survivorship, will present a teleconference titled "Survivors Too: Family, Friends and Loved Ones" on June 24 from 1:30 to 2:30 p.m. ET.
This is the third of a three-part telephone education workshop series called "The Sixth Annual Cancer Survivorship Series: Living With, Through, and Beyond Cancer."
Part I, "The Importance of Communicating with Your Doctor about Follow-Up Care," took place on April 22, and Part II, "Rediscovering Intimacy in Your Relationships Following Treatment," took place on May 13. Both workshops are archived on the CancerCare Web site.
No phone charges apply. However, pre-registration is required. To access the archive or to register, go to http://www.CancerCare.org/TEW.
| |
 |
 |

Defining Therapy for Recurrent Platinum-sensitive Ovarian Cancer
Name of the Trial
Phase III Randomized Study of Adjuvant Chemotherapy Comprising Carboplatin and Paclitaxel with Versus without Bevacizumab and/or Secondary Cytoreduction Surgery in Patients with Platinum-Sensitive Recurrent Ovarian Epithelial Cancer, Primary Peritoneal Cavity Cancer, or Fallopian Tube Cancer (GOG-0213). See the protocol summary at http://cancer.gov/clinicaltrials/GOG-0213.
 Principal Investigators
Principal Investigators
Dr. Robert Coleman, Dr. Scott Eisenkop, Dr. Deborah Armstrong, Dr. Thomas Herzog, and Dr. Paul Sabbatini, Gynecologic Oncology Group
Why This Trial Is Important
Ovarian cancer is one of the most common gynecologic cancers and is expected to strike more than 21,000 U.S. women in 2008. Primary peritoneal cancer and fallopian tube cancer are similar diseases that often respond to the same treatments used for ovarian cancer.
Typically, ovarian cancer is treated with surgery to remove the cancer, followed by combination chemotherapy with a platinum-containing drug (carboplatin or cisplatin) and a taxane, such as paclitaxel. Ovarian cancer that responds to chemotherapy with a platinum-containing drug is called platinum sensitive. Even if it recurs after treatment, platinum-sensitive ovarian cancer may respond again to treatment with a platinum-taxane combination.
While this common therapy does help some women live longer, doctors are eager to find better ways to treat recurrent ovarian cancer. One method under investigation is the addition of the biological agent bevacizumab to combination chemotherapy. Bevacizumab works by blocking the development of new blood vessels to tumors. Another approach, called secondary cytoreductive surgery, is performing another operation to remove the recurrent tumors.
In this phase III clinical trial, women with platinum-sensitive, recurrent ovarian epithelial, fallopian tube, or primary peritoneal cancer will be assessed first to determine whether or not they are candidates for secondary cytoreductive surgery. Surgical candidacy will be determined by whether or not there is a high likelihood of complete tumor removal, based on clinical evaluation and imaging. Women found to be candidates for this surgery will be randomly assigned to undergo it or not. These women will then be randomly assigned to receive combination chemotherapy with carboplatin plus paclitaxel or the same chemotherapy plus bevacizumab, followed by maintenance bevacizumab therapy after the chemotherapy is completed.
Women who are determined to not be candidates for secondary cytoreductive surgery at the start of the trial will immediately be randomly assigned to receive carboplatin plus paclitaxel chemotherapy or the same chemotherapy plus bevacizumab, which will also be followed by maintenance bevacizumab.
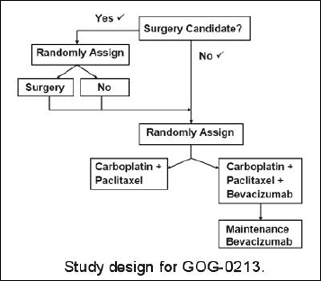
"At this point, the optimal therapy for women with recurrent, platinum-sensitive ovarian cancer is not well defined," said Dr. Coleman. "We have seen positive results from both of these experimental therapies in early stage clinical trials, and with this trial, we are hoping to establish their benefits for women with these cancers."
For More Information
See the lists of entry criteria and trial contact information at http://cancer.gov/clinicaltrials/GOG-0213 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |

Cancer.gov en Español Celebrates First Year, Looks Forward to Growth
An announcement last month by the U.S. Census Bureau noted that Hispanics and Latinos are not only the largest minority group in the country, but they are also the fastest growing. In anticipation of their health information needs, NCI launched 50 a Spanish-language Web site, Cancer.gov en español 51, in April 2007.
"Although Latinos in this country represent a diverse population with respect to cultures, Cancer.gov en español is an important step in the right direction to meet their information needs," says Nelvis Castro, associate director of multicultural and international communications in NCI's Office of Communications and Education (OCE).

The site has had strong reviews, including six NIH Plain Language Awards, an NIH Director's Award, and more than 140 articles publicizing the site across various national Spanish-language markets, including CNN en Español. After it launched, the number of visitors to NCI's Spanish-language Web pages jumped by 10 percent.
"It's not always easy to find relevant and appropriate cancer information in Spanish, but Cancer.gov en español offers this in one easy-to-access Web site," says Dr. Yvette Colón, director of education and internet services at the American Pain Foundation. "We provide several links to it from our Web site, and our Pain Information staff members regularly include it as a resource for callers."
By 2007, many NCI materials were already available in Spanish, notes José Acosta, a technical writer and editor in OCE. "What we needed to develop was a new Web site structure," he says, "one that would be proper for the cultural needs of Latinos."
Among the approximately 46 million Latinos living in the U.S., about half can speak English comfortably. Research shows, however, that Spanish messages have a stronger emotional impact on Latinos than those written or spoken in English. And even among second-generation immigrants, culture influences their preference for images and content that reflect their heritage.
NCI addressed these trends by designing a Web site for Latinos living in the U.S. who are confronting cancer personally, as family members of a cancer patient, or as health professionals seeking cancer research information in Spanish to provide to their patients.
"We're trying to address the myths and beliefs that exist within the Latino community around cancer right from the home page," says Anne Middleswarth, a Web content manager in OCE. Four homepage banners highlight cancer prevention, treatment, detection, and survivorship, and lead to content that corrects misunderstandings about the topics.
"It's become a tool for navigating the health care system," explains Silvia Inéz Salazar, a public health advisor in OCE. She points to the Spanish-language dictionary on the site, which contains more than 5,000 entries and allows people to toggle back and forth between the Spanish and English versions of cancer terms. She notes that both patients and health care providers have used this translation function to improve their communications.
Members of offices across NCI have helped with content development and promotion of Cancer.gov en español. For example, information specialists at the NCI Cancer Information Service call center in Miami, FL, which answers Spanish-language inquiries from the public, helped test the site before it went live, providing valuable feedback on navigation and the search engine.
The site will continue its expansion in 2008, with targeted development of new pages that reflect the areas of greatest interest and need among Spanish-speaking audiences.
—Brittany Moya del Pino
|
 |
|
Table of Links
| 1 | http://www.cancer.gov/cancertopics/druginfo/cetuximab |
| 2 | http://www.cancer.gov/cancertopics/druginfo/panitumumab |
| 3 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_061008/page2 |
| 4 | http://www.cancer.gov/cancertopics/druginfo/gemcitabinehydrochloride |
| 5 | http://www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Abstracts?&vmview=abst_d
etail_view&confID=55&abstractID=34749 |
| 6 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_061008/page3 |
| 7 | http://www.cancer.gov/cancertopics/druginfo/trastuzumab |
| 8 | http://www.abstract.asco.org/AbstView_55_34491.html |
| 9 | http://www.asco.org/ASCO/Abstracts+&+Virtual+Meeting/Abstracts?&vmview=
abst_detail_view&confID=47&abstractID=33250 |
| 10 | http://www.abstract.asco.org/AbstView_55_35260.html |
| 11 | http://www.abstract.asco.org/AbstView_55_34722.html |
| 12 | http://ctep.cancer.gov |
| 13 | http://www.abstract.asco.org/AbstView_55_30338.html |
| 14 | http://www.cancer.gov/newscenter/pressreleases/AvastinLung |
| 15 | http://www.cancer.gov/cancertopics/druginfo/bevacizumab |
| 16 | http://www.cancer.gov/cancertopics/druginfo/zoledronicacid |
| 17 | http://www.abstract.asco.org/AbstView_55_35897.html |
| 18 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_040108/page6 |
| 19 | http://www.cancer.gov/cancertopics/druginfo/tamoxifencitrate |
| 20 | http://www.cancer.gov/cancertopics/druginfo/anastrozole |
| 21 | http://www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Abstracts?&vmview=abst_d
etail_view&confID=55&abstractID=35983 |
| 22 | http://medicine.plosjournals.org/perlserv/?request=get-document&doi=10.1371/jou
rnal.pmed.0050123 |
| 23 | http://edrn.nci.nih.gov |
| 24 | http://emice.nci.nih.gov |
| 25 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_042908/page2 |
| 26 | http://healthservices.cancer.gov/seermedicare |
| 27 | http://www.cancer.gov/cancertopics/druginfo/erlotinibhydrochloride |
| 28 | http://dccps.nci.nih.gov |
| 29 | http://cancergenome.nih.gov |
| 30 | http://www.cancer.gov/ncicancerbulletin/cancerbulletin/page4 |
| 31 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_042908/page4 |
| 32 | http://dtp.nci.nih.gov |
| 33 | http://iotftraining.nci.nih.gov |
| 34 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_021908/page4 |
| 35 | http://dcp.cancer.gov/programs-resources/programs/ccop |
| 36 | http://www.cancer.gov/cancertopics/factsheet/NCI/clinical-trials-cooperative-gr
oup |
| 37 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_062607/page2 |
| 38 | http://ccr.cancer.gov/staff/staff.asp?profileid=11782 |
| 39 | http://ccr.cancer.gov/labs/lab.asp?labid=753 |
| 40 | http://dtp.nci.nih.gov/docs/raid/raid_index.html |
| 41 | http://www.cancer.gov/cancertopics/factsheet/Detection/PLCOOvarianFactSheet |
| 42 | http://preview.cancer.gov/ncicancerbulletin-archive |
| 43 | http://prevention.cancer.gov |
| 44 | http://prevention.cancer.gov/programs-resources/groups/cb |
| 45 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=45290 |
| 46 | http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=45661 |
| 47 | http://www.fda.gov/medwatch/safety/2008/safety08.htm#TNF |
| 48 | http://www.fda.gov/medwatch/safety/2008/safety08.htm#Regranex |
| 49 | http://ccr.cancer.gov/labs/lab.asp?labid=107 |
| 50 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_040307/page3 |
| 51 | http://www.cancer.gov/ESPANOL |
|
 |