
New Chemo Agents Travel Winding Road
At a time when there is no shortage of studies that identify potential molecular targets for new cancer therapies and many pharmaceutical and biotechnology companies are banking on targeted cancer agents to ensure future success, cytotoxic chemotherapy still represents the foundation of treatment for the vast majority of cancers.
In fact, a number of pharmaceutical companies are busily developing new chemotherapeutic agents.
Among those under development, a class of drugs known as epothilones has generated intense interest because of promising results from preclinical studies and early-phase clinical trials, and because they work similarly to a class of highly effective chemotherapy drugs, the taxanes, but don't require premedication with steroids to fend off allergic reactions. In June, the U.S. Food and Drug Administration (FDA) granted priority review to Bristol Myers Squibb's application to market ixabepilone, the epothilone furthest along in clinical testing, for the treatment of metastatic breast cancer. Priority review means FDA intends to complete the review within 6 months.
 |
 |
Watch Your E-Mail Box! |
 |
|
Keep an eye out for an e-mail later this week asking you to complete an online survey about the NCI Cancer Bulletin.
By completing this short questionnaire, you'll help us to better meet the needs of our readers. Your feedback is vital in shaping future issues of the Bulletin.
All survey responses are confidential and respondents can choose to answer or skip any questions in the survey. For more information, please contact Nina Goodman at goodmann@mail.nih.gov or 301-435-7789. |
|
It's unclear at this point, though, what FDA approval of ixabepilone would mean for the epothilones' long-term prospects. Treatment options for some of the cancers in which epothilones are being studied have expanded dramatically. And, as a handful of researchers have argued, in the absence of tests that indicate which patients are the best candidates for which treatments, it may prove difficult for any new chemotherapeutic agent that doesn't demonstrate clear superiority to other available agents to establish a foothold in the clinic.
In August, results of five phase II clinical trials testing ixabepilone were published in the Journal of Clinical Oncology (JCO) - four trials in metastatic breast cancer and one in advanced non-small-cell lung cancer (NSCLC). In all five trials, the drug showed comparable efficacy to what has historically been seen with the standard-of-care drugs, including taxanes such as paclitaxel and docetaxel, that are the cornerstones of treatment for metastatic breast cancer.
And, as important, it had what's considered an "acceptable" toxicity profile - one that produced toxicities that, although not negligible, were manageable and didn't prohibit the drug's use in most patients for long enough to provide a clinical benefit.
"The evidence to date on ixabepilone [to treat metastatic breast cancer] suggests that it is extremely effective either as a first-line therapy or after patients have undergone multiple treatments, especially with an anthracycline or after taxanes," explains Dr. Sandra Swain, medical director of the Washington Cancer Institute in Washington, DC, who has led several phase II trials involving ixabepilone.
Ixabepilone also produced favorable results in one of two ongoing phase III breast cancer trials in which it is being investigated, comparing it plus capecitabine to capecitabine alone in patients with metastatic breast cancer who have progressed after standard treatments. Presented at the American Society of Clinical Oncology annual meeting in June, the results of the trial were not staggering - a 1.5 month improvement in progression-free survival and a 2.5-fold increase in overall response rate - but the improvements compared favorably enough with the current standard to secure an FDA priority review.
Although they are not generally considered "targeted" drugs, most chemotherapeutic agents do have a target: rapidly dividing cells. In the epothilones' case, just like the taxanes and the vinca alkaloids, another class of chemotherapy agents, they target a component of cells' cytoskeleton known as microtubules, which are critical to cell division. All three types of agents work by stabilizing microtubules; this disrupts the process of cell division (and thus, further proliferation) and eventually leads to cancer cell death.
"We know the tubulins have been a good target," says Dr. Edith Perez, director of the Breast Cancer Program at the Mayo Clinic Jacksonville and investigator on one of the JCO-published ixabepilone trials.
But the epothilones, of which there are four being tested in phase II or III trials (see table), "appear to bind in a different location compared with other tubulin-targeting agents," she adds. That may explain why these drugs have shown activity in patients who have developed resistance to other tubulin-targeting agents like
the taxanes.
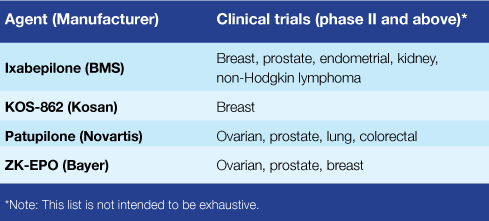
That was the case in four of the five trials published last month in JCO (the fifth used ixabepilone as a first-line treatment). In the advanced NSCLC trial, ixabepilone demonstrated results comparable to those seen with three other drugs already FDA-approved for patients whose tumors are no longer responding to the first-line treatment.
And therein lies part of the difficulty for any new chemotherapeutic agent, notes Dr. Alan Sandler, medical director of Thoracic Oncology at Vanderbilt University and co-investigator on the trial.
"It's not like the old days where you didn't have anything for these patients," he says. "We now have three agents for second line, two chemotherapeutic and one targeted agent."
In the case of ixabepilone for the treatment of NSCLC, he says, it's still unclear where it can or will go from here. For example, there is potential in the second-line arena because
two of the approved second-line agents, pemetrexed and docetaxel, are now being investigated as first-line treatments. If they succeed, that could free up space in the second-line arsenal for an agent like ixabepilone that has already shown some activity in that setting.
If approved by the FDA, Dr. Perez says, it appears as if ixabepilone at least would present new options. In the case of the phase II trial in which she was involved, for example, there is no approved therapy for the type of patients it enrolled, all of whom were resistant to anthracyclines, taxanes, and capecitabine.
"These patients are now treated with unapproved drugs," she notes, "so it was nice to demonstrate that ixabepilone has clear activity, thus addressing an unmet patient need."
Information from NCI’s PDQ Cancer Clinical Trials Registry about epothilone clinical trials can be found at: Ixabepilone clinical trials, Patupilone clinical trials, and ZK-EPO clinical trials.
—Carmen Phillips
|