 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Hysterectomy Surveillance --- United States, 1994--1999Homa Keshavarz, M.D.1,2 Abstract Problem/Condition: Hysterectomy is the second most frequently performed surgical procedure, after cesarean section, for women of reproductive age in the United States. Approximately 600,000 hysterectomies are performed annually in the United States, and approximately 20 million U.S. women have had a hysterectomy. Reporting Period Covered: This report covers data from 1994 through 1999 Description of System: Estimates of the population of U.S. female, civilian residents were used to compute rates for this study. Population denominators were obtained from the U.S. Bureau of the Census. The National Hospital Discharge Survey (NHDS) was the data source for this report. NHDS is conducted by CDC's National Center for Health Statistics. NHDS is an annual, multistage probability sample of short-stay patients (those hospitalized <30 days) discharged from nonfederal hospitals in the United States. Results and Interpretation: From 1994 through 1999, an estimated 3,525,237 hysterectomies were performed among U.S. women aged >15 years, and the overall hysterectomy rate for U.S. female, civilian residents was 5.5 per 1,000 women. Although statistically significant increases for hysterectomy rates were observed from 1994 (5.1/1,000) through 1998 (5.8/1,000), the increase was limited and the curve remained nearly flat. Women aged 40--44 years had a significantly higher hysterectomy rate compared with any other age group. During the study period, 52% of all hysterectomies were performed among women aged <44 years. In addition, hysterectomy rates per 1,000 in women aged 45--54 years increased significantly, from 8.9 in 1994 to 10 in 1999. The overall hysterectomy rate for women living in the South was 6.5 per 1,000, which was significantly higher than the rate among women who lived in either the Northeast (4.3) or the West (4.8) but not significantly higher than the rate among women who lived in the Midwest (5.4). Uterine leiomyoma, endometriosis, and uterine prolapse were the most frequent diagnoses for women aged >15 years. The percentage of uterine leiomyoma as a primary diagnosis for hysterectomy increased 10.2% for white women, 7.8% for black women, and 23% for women of other races. Among women who had a hysterectomy during the study period, 55% also had a bilateral oophorectomy. The proportion of all vaginal hysterectomies with concomitant laparoscopy (LAVH) increased significantly, from 13% in 1994 to 28% in 1999. During this same period, the percentage of cases of LAVH with concomitant bilateral oophorectomy increased significantly, from 20.4% in 1994 to 42.5% in 1999. Public Health Actions: Continued monitoring of hysterectomy trends will be necessary to evaluate differences in hysterectomy rates by age, most commonly associated diagnoses, whether leiomyomata as a primary discharge diagnosis continues to increase, and whether the increase in LAVH that occurred during the previous decade continues. IntroductionAfter cesarean section, hysterectomy is the second most frequently performed major surgical procedure for women of reproductive age in the United States. Approximately 600,000 hysterectomies are performed annually in the United States, and an estimated 20 million U.S. women have had a hysterectomy (1,2). The previous summary of hysterectomy surveillance in the United States described trends during 1980--1993 (3). Hysterectomy rates declined during 1980--1986 and increased steadily during 1988--1993. However, during the latter period, the rates of vaginal hysterectomy, concomitant bilateral oophorectomy, and laparoscopically assisted vaginal hysterectomy (LAVH) increased (3). To monitor recent increasing trends in the number of hysterectomies performed in the United States, data from the 1994--1999 National Hospital Discharge Survey (NHDS) were analyzed. Methods Study Population, NHDS Data, and Sampling The population studied comprised women aged >15 years who had a hysterectomy during 1994--1999. The estimated population of U.S. female, civilian residents was used to compute rates for this study. The population sample was obtained from the U.S. Bureau of the Census and included in a documentation package from NHDS (4). Data from the NHDS conducted by CDC's National Center for Health Statistics were analyzed. NHDS is an annual, multistage probability sample of short-stay patients (those hospitalized <30 days) discharged from nonfederal hospitals in the United States. NHDS uses a modified three-stage probability design. The stages are 1) primary sampling units (PSUs), 2) hospitals within PSUs, and 3) discharges within hospitals. The modification was characterized by the selection with certainty of the largest PSUs and hospitals (5). Definition of Variables Age The study participants were grouped by ages 15--24, 25--29, 30--34, 35--39, 40--44, 45--54, and >55 years. This strategy ensured stable estimates in each age group and matched the age groups used in the previous report (3). Race Participants were also grouped by race into white, black, or other category, on the basis of medical record abstraction. The other category included Asians, Pacific Islanders, American Indians, Alaskan Natives, and other races not already specified. Missing race information ranged from 17.1% in 1995 to 20.5% in 1997. In this report, patients of unknown race were allocated proportionate to the racial distribution of known cases. When examining proportions, the point estimates based on proportional allocation would not differ from those based only on known race. However, if rates were calculated where the numerators were based only on patients with known race, rates would be understated in all cases. Geographic Region The geographic areas included in the study were based on the U.S. Bureau of the Census definition of the four regions of the United States: Northeast (Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont); Midwest (Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin); South (Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia); and West (Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming). Medical Classification Estimates of diagnoses and procedures in NHDS were derived from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Women with codes 68.3--68.5 were classified as having undergone hysterectomy. ICD-9-CM code 65.5 or 65.6 identified concomitant bilateral oophorectomy; ICD-9-CM code 54.21 indicated laparoscopy as a procedure before 1997; and the new ICD-9-CM code 68.51 identified LAVH since 1997. NHDS also provides information regarding whether the surgical approach was an abdominal or vaginal hysterectomy. Radical hysterectomy, ICD-9-CM 68.6 or 68.7, and pelvic evisceration performed for advanced pelvic cancer treatment (ICD-9-CM 68.8) were excluded. Because NHDS includes seven diagnostic codes, the following algorithm was used to define the leading cause of hysterectomy: 1) cancer was assigned as the leading cause of hysterectomy if reproductive tract cancer or debulked cancer of the urinary or intestinal tract were listed as a diagnosis; 2) precancerous condition was assigned as the cause of hysterectomy if a precancerous condition was listed as a diagnosis (e.g., endometrial hyperplasia or carcinoma in situ of the cervix); 3) uterine leiomyoma, endometriosis, or uterine prolapse, whichever was listed first, was assigned as the primary diagnosis if cancer or a precancerous condition was not listed; and 4) "other" was assigned as the primary diagnosis for the remaining recorded diagnoses (6,7). Statistical Analysis Rates per 1,000 women aged >15 years in the U.S. civilian resident population were computed. SUDAAN software was used to account for the complex survey design (8). Data were weighted to produce national estimates. Because discharges were reallocated in cases where race was not reported, SUDAAN could not be used to compute standard errors directly for race-specific estimates. Therefore, the relative standard error computed with SUDAAN for participants of known race was applied to each reallocated total. Trends in both discharge rates and proportions were computed by using SUDAAN and SAS (8,9). The yearly estimates and corresponding covariance matrices were computed with SUDAAN and then put into SAS's PROC CATMOD, where weighted least-squares estimation was used to test for trends over time. Results From 1994 through 1999, an estimated 3,525,237 hysterectomies were performed in the United States for women aged >15 years. The overall hysterectomy rate was 5.5 per 1,000 women per year (95% confidence interval [CI] = 5--5.9) during the 6-year study period (Table 1). Although statistically significant increases were observed in rates from 1994 through 1998 (p for trend = 0.04), the increase was limited, and the overall curve during this period was nearly flat. Rates appeared to decline in 1999 (Figure 1). The overall estimated hysterectomy rate for blacks was 6.2 per 1,000 and 5.9 per 1,000 for other races. The hysterectomy rate for whites was 5.3 per 1,000. Differences in overall rates by race were not statistically significant (Table 2). However, differences in hysterectomy rates among black and white women aged 35--39 and 40--44 years were statistically significant (p<0.05). Women aged 40--44 years had a significantly higher rate of hysterectomy (11.7; 95% CI = 10.7--12.6) than did any other age group. During the study period, 52% of all hysterectomies were performed among women aged <44 years. Hysterectomy rates among women aged 45--54 years increased significantly, from 8.9 per 1,000 in 1994 to 10 per 1,000 in 1999 (p for trend = 0.009) (Figure 2). The highest hysterectomy rate was 16.8 per 1,000 in black women aged 40--44 years. White women aged 40--44 years had a hysterectomy rate of 10.8 per 1,000, and women of other races aged 40--44 years had a hysterectomy rate of 12.4 per 1,000 (Table 2). The overall hysterectomy rate for women living in the South was 6.5 per 1,000 (95% CI = 5.6--7.5), which was significantly higher than the rate for women living in either the Northeast (4.3; 95% CI = 3.7--5) or the West (4.8; 95% CI = 4.2--5.4), but not significantly higher than the rate among women who lived in the Midwest (5.4; 95% CI = 4.6--6.1) (Figure 3). The average age at which women in the South underwent a hysterectomy was 44.5 years (95% CI = 44--45), which was significantly younger than the average age of women living in all other regions (p<0.05). The highest average age was 49.1 years (95% CI = 48.4--49.9) for women in the Northeast. Uterine leiomyoma, endometriosis, and uterine prolapse were the most frequent diagnoses for women undergoing hysterectomy and accounted for 73% of all hysterectomies during 1994--1999. The percentage of hysterectomies with a diagnosis of uterine leiomyoma was 68% among black women, 33% in white women, and 45% among women of other races (Table 3). Women in the >55-year age group had higher numbers of diagnoses of hyperplasia, cancer, and uterine prolapse, whereas women in the age groups from 30 through 54 years had higher numbers of cases of leiomyomata and endometriosis (Table 4). From 1994 through 1999, the percentage of cancer as a primary diagnosis for hysterectomy significantly decreased by 20% (p for trend = 0.01), and uterine leiomyoma increased significantly by 11% (p for trend = 0.02) (Figure 4). The percentage of uterine leiomyoma as a primary diagnosis for hysterectomy increased 10.2% among white women (p for trend = 0.20), 7.8% among black women (p for trend = 0.08), and 23% among women of other races (p for trend = 0.42). Abdominal hysterectomies were performed more frequently than vaginal hysterectomies for all races. Black women were more likely to undergo abdominal hysterectomy than white women; conversely, white women were more likely to undergo vaginal hysterectomy than black women (Figure 5). The higher prevalence of leiomyoma among black women might contribute to the higher proportion of abdominal hysterectomies in this group. Among women who had a hysterectomy during 1994--1999, 55% had a bilateral oophorectomy. Among women aged 15--44 years who underwent a hysterectomy, approximately one third (40%) had a concomitant bilateral oophorectomy, and these women can be considered to have undergone surgical menopause. The percentage of bilateral oophorectomy was highest (75%) for women aged 45--54 years. The proportion of hysterectomies with concomitant bilateral oophorectomies tended to increase with increasing age (Figure 6). Women who had a bilateral oophorectomy were 4.4 times as likely to have an abdominal, rather than a vaginal hysterectomy. Seventy-one percent of bilateral oophorectomies were performed on women whose primary diagnoses were benign (e.g., leiomyoma, endometriosis, or uterine prolapse). The trends for all hysterectomies performed vaginally and the percentage of all vaginal hysterectomies with concomitant bilateral oophorectomies remained stable during 1994--1999 (Figure 7). However, the proportion of all vaginal hysterectomies with concomitant LAVH increased significantly, from 13% in 1994 to 28% in 1999 (p for trend <0.0001). Further analyses were performed to assess LAVH trends. During the study, the number of vaginal hysterectomies with concomitant bilateral oophorectomies remained stable (Figure 8). However, the percentage of vaginal hysterectomies with concomitant bilateral oophorectomies that were assisted by laparoscopy increased significantly, from 20.4% in 1994 to 42.5% in 1999 (p for trend <0.0001). Discussion Study findings indicated a limited, yet significant increase in the rate of hysterectomies during 1994--1998. From 1994 through 1999, one in every nine women aged 35--45 years had a hysterectomy. Women aged 40--44 years had a significantly higher rate of hysterectomies than all other age groups. The most common primary diagnoses for a hysterectomy were uterine leiomyoma, endometriosis, and uterine prolapse (3). Approximately one half of the women who had a hysterectomy also had a bilateral oophorectomy. Rates of vaginal hysterectomies remained stable, although the proportion of women who had an LAVH increased significantly by >2-fold. Furthermore, the proportion of vaginal hysterectomies with concomitant oophorectomies performed that were assisted by LAVH approximately doubled during 1994--1999. The findings in this report confirm and extend those noted in previous reports: rates of hysterectomy were higher among black women than among white women. Although differences in overall hysterectomy rates among black and white women were not significant, differences in age-specific rates (35--39 years and 40--44 years) were significant (3,10--13). Rates of hysterectomy were also higher among women aged 40--44 years (3,10,12--15) and women living in the South (3,10,12--14,16). The availability of obstetricians and gynecologists, the number of hospital beds per capita, and the type of health-care insurance available might vary by region; these factors might influence regional variation (17,18). Uterine leiomyoma, which is the most common uterine tumor, was the most frequent diagnosis associated with hysterectomy. In addition, the overall rate of hysterectomy attributable to uterine leiomyoma during 1994--1999 (2.1 per 1,000) increased by 17% over the rate for the previous study period (1988--1993: 1.8 per 1,000) (3). More widespread availability of ultrasound in obstetrician-gynecologist clinics might have increased the diagnosis of uterine leiomyoma (19). Findings in this report do not include data to help explain reasons for observed racial differences in the occurrence of leiomyoma or hysterectomy rates among women aged 30--44 years. However, family history has been associated with leiomyoma, prompting an ongoing search for genetic and other potential risk factors shared by women from the same families (20). In contrast with leiomyomas, cancer as a primary diagnosis for hysterectomy decreased by 21% during the study period. A recent report demonstrates that the reduction occurred primarily in cervical cancers as a primary diagnosis for hysterectomy. Data in this report indicate that the reduction might be a consequence of increased detection of precancerous lesions by Papanicolaou smear programs (21) and of changes in choice of treatment for early lesions (22,23). Although trends for bilateral oophorectomy remained stable during 1994--1999, the percentage of hysterectomy with concomitant bilateral oophorectomy more than doubled from 1965 (25%) to the time of this evaluation (55%) (15). More than one half of hysterectomies were accompanied by bilateral oophorectomies, the majority of which were performed abdominally. An evaluation of the indications for oophorectomies is beyond the scope of this report. When vaginal hysterectomies included concomitant bilateral oophorectomy, increases in the use of laparoscopic assistance were observed. These increases might indicate a preference by surgeons for increased visibility of the ovaries when oophorectomy is performed (24). Bilateral oophorectomy might have diverse implications for women's health (e.g., ovarian cancer prevention, need for hormone replacement therapy). According to the analysis in this report, LAVH approximately doubled from 1994 to 1999, although rates of vaginal hysterectomy remained stable. Since 1989, when LAVH was first introduced, to 1999, the percentage of all hysterectomies that were LAVHs increased significantly from 1% to 28%. LAVHs are controversial, partly because of cost concerns (25,26), although LAVH-associated benefits might include shorter hospital stays, less pain, more rapid recovery, and fewer complications. These benefits are relevant, however, only when LAVH replaces abdominal hysterectomy, rather than substituting for unassisted vaginal hysterectomy (2,27). Limitations Several limitations exist in the data presented in this report. For example, the denominator included all U.S. women, rather than only those women who had not previously undergone a hysterectomy, resulting in an underestimate of the true rates of hysterectomy among those at risk in the United States. This limitation, however, should not affect the ability to detect trends over time as long as rates remain stable. Another limitation was the assumption that the racial distribution of discharges where race was not reported is the same as for discharges where race was reported. This assumption is tenuous because a recent evaluation indicates that race data might be more likely to be missing for white women (28). Furthermore, random assignment would attenuate any differences by race. Therefore, the race-specific estimates presented in this report should be interpreted with caution. In addition, pathology reports were not available to help confirm indications for the performance of hysterectomy. Finally, data regarding socioeconomic status and parity were not available. In summary, the data demonstrate limited but significant increases in hysterectomy rates from 1994 through 1998, followed by a decrease in 1999. During the study period, significant increases were observed in the occurrence of leiomyomas as a primary diagnosis for hysterectomy, whereas significant decreases occurred in the reporting of cancer as a primary diagnosis. Continued monitoring of hysterectomy trends will be necessary to evaluate differences in hysterectomy rates by age, most commonly associated diagnoses, whether leiomyomata as a primary discharge diagnosis continues to increase, and whether the increase in LAVH that occurred during the previous decade will continue. References
Table 1 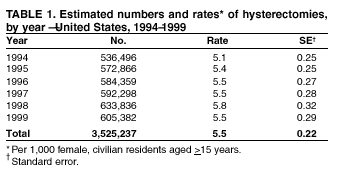 Return to top. Figure 1 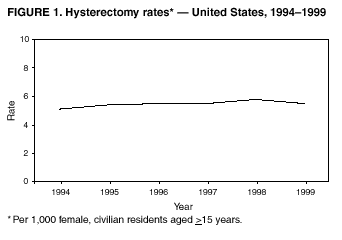 Return to top. Table 2 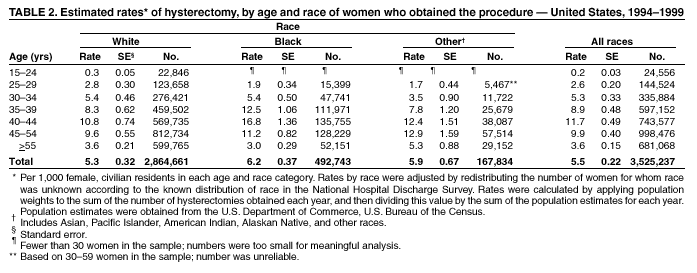 Return to top. Figure 2 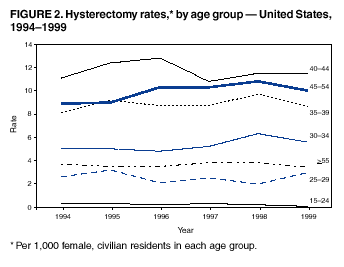 Return to top. Table 3 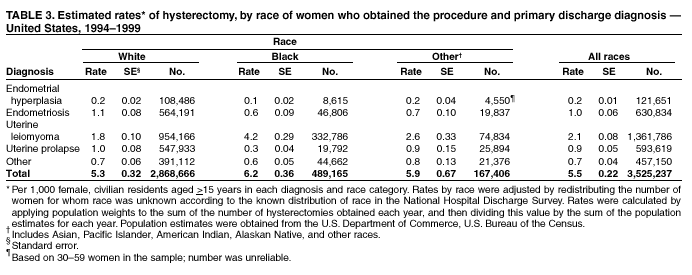 Return to top. Figure 3 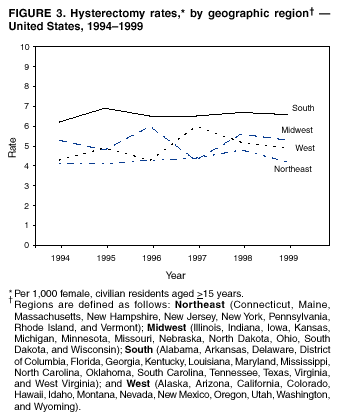 Return to top. Table 4 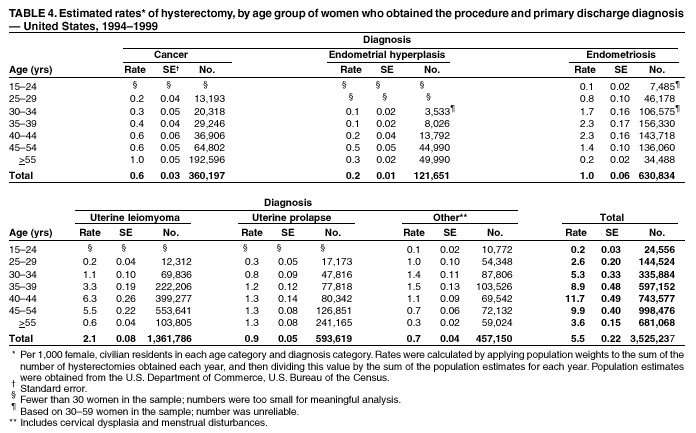 Return to top. Figure 4 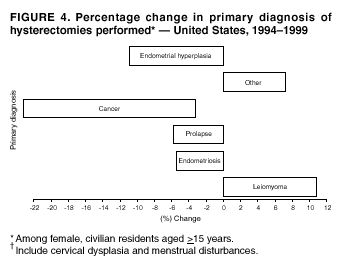 Return to top. Figure 5 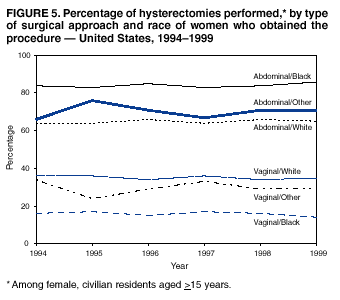 Return to top. Figure 6 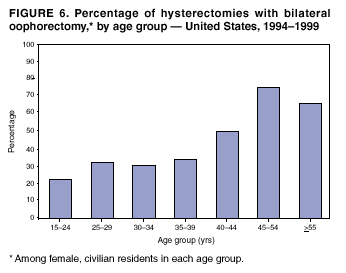 Return to top. Figure 7 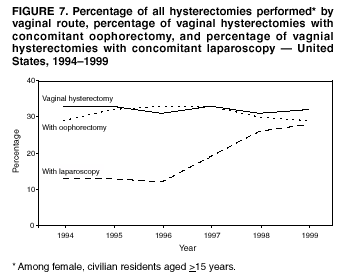 Return to top. Figure 8 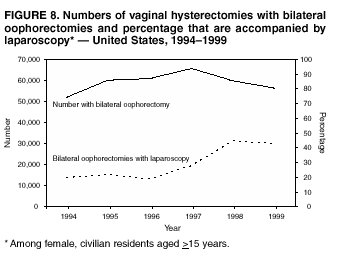 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/2/2002 |
|||||||||
This page last reviewed 7/2/2002
|