 |
|
SPECIAL Issue |
November
2006
|
|
R01 applications require
electronic submission
for
February 2007 and beyond.
New form...new process…find out
how! |
|
|
|
NEWS
FROM THE DIRECTOR OF OER: NIH WILL BEGIN ACCEPTING
R01 GRANT APPLICATIONS ELECTRONICALLY ON FEBRUARY
5, 2007 |
|
The Electronic Application Submission
Process |
|
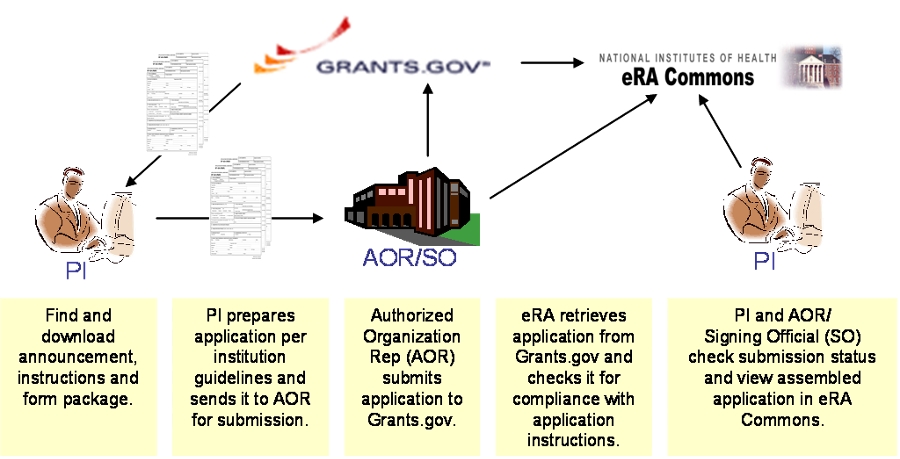 |
|
(Click on picture for
larger view) |
Dear Extramural Community, PIs, Administrators, et
al.:
On February 5, 2007, NIH will begin accepting
electronic applications of R01 Research Project
Grants, our most heavily used mechanism. Both NIH
and applicant institutions have been preparing for
this event for over a year. We have built systems;
developed new processes to prepare, route, and
process electronic applications; and we have
dedicated numerous
presentations, meetings,
emails, Web sites, newsletters and outreach
materials to this topic.
Since I announced in August 2005, NIH’s plans to
move to electronic submission, we have (with, I
will admit, a few bumps along the way)
transitioned more than 14 of our smaller grant
programs and have received nearly 18,000 unique
electronic applications. The upcoming electronic
submission of R01s likely will set new submission
records at both Grants.gov and eRA Commons. To
handle this anticipated increased demand, we have
changed our standard receipt dates for
applications so that we spread the workloads
on systems within NIH and within the business
offices of applicant institutions. The receipt
date changes, in combination with recent system-performance improvements, have minimized capacity
concerns and we are well positioned to handle the
increased load.
I want you to know that if problems do arise in
spite of all our preparation, we have contingency
plans to address them, and I assure you that NIH
will not penalize you for a Grants.gov or eRA
Commons system issue.
There is a learning curve associated with using
the new submission process and forms. NIH is
hosting a
training event available through NIH VideoCast
on December 5, 2006 that will be a great
introduction to these changes. Although the
hands-on workshops offered in conjunction with
this training event are full, additional hands-on
training opportunities will be available including
those at
NIH Regional Seminars on Program Funding and
Grants Administration. I also encourage you to
take advantage of the available online
training resources, including NIH VideoCasts,
tutorials, videos and the eRA Commons demo facility for
hands-on experience in a non-production
environment. My team will continue to expand the
training resources as we move towards the February
R01 receipt date.
NIH staff and I recognize that the electronic
submission process is complex, requires advance
preparation, and represents a significant change
in how an application is written and how it is
submitted. eSubmission is in many cases redefining
the relationship between a PI and his/her
institutional administrator. We recognize that
these changes may be provoking some anxiety. It is
my hope that the articles in this special edition
of the NIH Extramural Nexus, which focus on
electronic submission of the R01 grant
application, will demystify the electronic
submission process and help relieve the anxiety
that some of you may be feeling.
I really appreciate all the feedback that we have
received from you—applicants and applicant
institutions. Your feedback has helped us make
significant improvements in the system, and I
encourage you to continue to speak up. Please feel
free to write to me at
DDER@NIH.gov
with comments or questions.
 |
Norka Ruiz Bravo, Ph.D.,
Director, Office of Extramural Research and NIH Deputy Director for
Extramural Research |
 Back
to top Back
to top
|
|
ELECTRONIC SUBMISSION OF R01 GRANT
APPLICATIONS: THE PROCESS |
|
The
NIH Electronic Submission of Grant
Applications Web site contains detailed
instructions, tips and resources to walk you
through the
electronic application submission process:
|
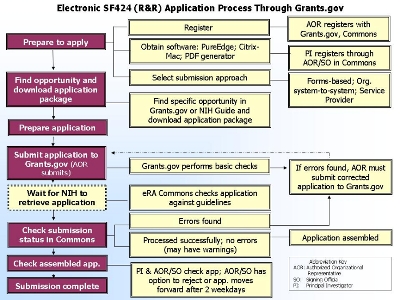 |
|
(Click on picture for
larger view) |
 Back
to top Back
to top
|
|
NIH
WILL NOT PENALIZE YOU FOR GRANTS.GOV OR eRA
COMMONS SYSTEMS ISSUES |
|
 The forms are different. The
submission method has changed. Some things,
however, remain the same—it is still about the
science and getting the best applications in the
door and ready for review. So, if you follow the application guide and opportunity instructions
and do everything you can to successfully submit
your application on time, but then cannot complete the
submission because of a Grants.gov or eRA Commons
system failure do not despair. Help
is available and NIH will ensure
that you are not penalized for a Grants.gov or eRA
Commons system problem. The forms are different. The
submission method has changed. Some things,
however, remain the same—it is still about the
science and getting the best applications in the
door and ready for review. So, if you follow the application guide and opportunity instructions
and do everything you can to successfully submit
your application on time, but then cannot complete the
submission because of a Grants.gov or eRA Commons
system failure do not despair. Help
is available and NIH will ensure
that you are not penalized for a Grants.gov or eRA
Commons system problem.
What should you do if you have a
problem? Immediately
contact the
eRA Commons help desk to report the
issue. The help desk can be busy around
submission deadlines so take advantage of the
Web
ticketing option to avoid telephone delays and ensure
the details of your issue are captured correctly.
As soon as the help desk staff confirms a system
issue, they will
document the issue and continue to work with you
until the problem is resolved.
System issues that prevent
application submission are fairly uncommon, but
they can and do occur. During our first electronic
submission rounds, a number of application
submissions were unsuccessful due to system
issues. At that time, most of the issues
were coding errors that prevented
correct information from being accepted. With the experience gained from processing nearly 18,000 unique applications, we have seen a significant drop in the number of coding issues. Currently, only a handful of application submissions encounter system issues.
No application that has been brought to the attention of the eRA Commons help desk with a confirmed system issue has missed
its review cycle due to a system issue.
All system issues that we have
experienced to date have affected individual or
small subsets of applications. NIH, however,
does have contingency plans in place to handle larger
system issues encountered close to standard
submission deadlines. Since on-time submission is
dependent on receipt of applications to Grants.gov by
5:00 p.m. local time, it is Grants.gov system
issues that are most problematic for applicants.
NIH's contingencies provide for extending
submission dates when Grants.gov is unavailable
for a significant period of time leading up to a
deadline. The
NIH Guide,
Electronic Submission
Program email lists and
Electronic Submission Web site will
be the primary vehicles used to communicate any
deadline extensions. If eRA Commons experiences a
significant interruption in service just after a
submission deadline, the error-correction window
may be extended to provide applicants with the
necessary time to check submission status, address
errors and view their corrected applications. The
Electronic Submission email lists and Electronic
Submission Web site will be the primary vehicle
used to communicate error-correction window
extensions.
To provide a fair and competitive
process, all applicants must follow the same rules
for application submission. The most common reason
for unsuccessful application submissions is
because the applicant did not follow the
application guide and funding opportunity
instructions. Please be aware that if your
submission failed to complete because you did not
follow all the instructions, NIH is under no
obligation to accept your late application.
There is a learning curve involved
with submitting electronic applications. Start the
submission process early, read and follow all
instructions carefully, subscribe to the
electronic mailing lists
and consult the NIH Electronic Submission Web site
or support desk when you have questions or
concerns.
 Back
to top Back
to top
|
|
UNSOLICITED
APPLICATIONS: USE "PARENT" ANNOUNCEMENTS |
 NIH’s commitment to unsolicited,
investigator-initiated research is deep and
longstanding. Investigators are the innovators of
the future—they bring fresh ideas and technologies
to existing biomedical research problems and they
pioneer new areas of investigation. NIH-funded
researchers are essential to the success of this
country’s biomedical research enterprise. NIH’s commitment to unsolicited,
investigator-initiated research is deep and
longstanding. Investigators are the innovators of
the future—they bring fresh ideas and technologies
to existing biomedical research problems and they
pioneer new areas of investigation. NIH-funded
researchers are essential to the success of this
country’s biomedical research enterprise.
NIH’s interest in pioneering new
areas of investigation is stronger than ever. The
implementation of
“Parent” Funding Opportunity
Announcements (FOAs) is a strategic part of this
interest. To apply for a grant from NIH, all
applications now must be submitted in response to
an FOA. Parent FOAs are designed to provide a
solicitation mechanism for unsolicited,
investigator-initiated applications. Responding to
a Parent FOA ensures that the correct application
package is used by applicants, and enables NIH to receive the
application through
Grants.gov.
Parent Announcements are NIH-wide, but some NIH
Institutes may limit their participation, so check
the announcement's statement of interest. For
institute-specific opportunities in your area of
science, search the
NIH Guide for Grants and
Contracts. There may be an FOA published by an
NIH Institute or Center that is searching specifically
for research that you are interested in pursuing.
We have received feedback from the applicant
community that the use of Program Announcements
(PAs) for the unsolicited, investigator-initiated
opportunities does not reflect correctly the
spirit of unsolicited research. Further, the “PA”
acronym found in the announcement number can be
seen as standing
for Program Announcement or Parent Announcement, adding an additional layer of confusion for
applicants searching for Parent Announcements.
Although NIH cannot address this issue for the
initial R01 submission rounds, we are developing plans
to address it in the future.
 Back
to top Back
to top
|
|
NIH
TRAINING: PREPARING FOR NIH ELECTRONIC GRANT
APPLICATION |
 NIH
will hold a presentation on December 5, 2006,
to prepare the applicant community for the
upcoming transition of R01s to electronic
submission. The training presentation will be held
at the Natcher Conference Center in Bethesda,
Maryland, and online via
NIH VideoCast in the morning with a repeat session in the afternoon. NIH
will hold a presentation on December 5, 2006,
to prepare the applicant community for the
upcoming transition of R01s to electronic
submission. The training presentation will be held
at the Natcher Conference Center in Bethesda,
Maryland, and online via
NIH VideoCast in the morning with a repeat session in the afternoon.The
presentation will include an overview of the electronic
submission process, a walk through the SF424
(R&R) grant application form, and “Lessons Learned” presented by a panel
of eSubmission experts. There also will be a
question-and-answer
session with the panel at the close of the
presentation.
Registration is required but the training is
offered free of charge. The training will be
archived for later viewing.
Presentation: Tuesday, December 5, 2006, Natcher
Conference Center, Main Auditorium, NIH Main
Campus, Bethesda, Maryland (onsite and via
VideoCast).
Click here for more details and registration
information.
 Back
to top Back
to top |
|
NIH
ON-TIME SUBMISSION FLEXIBILITY DURING THE eSUBMISSION
LEARNING
CURVE |
 All
NIH grant applications are required to be
submitted on time. Eventually, on time will mean
that a “clean” application (i.e., passed
Grants.gov and eRA Commons validations without
errors) has been submitted to Grants.gov by 5:00
p.m. local time on the receipt date. However, NIH
has provided some flexibility for the first few
submission dates of grant programs transitioning
to electronic submission. Applicant institutions
can submit changed/corrected applications in the
week (5 business days) following the submission deadline, provided
the changes made are needed to correct errors
encountered during the eRA business rule
validation process. This is not for tweaking the
content of a research plan, but for working
through specific errors on other fields of the
forms. All
NIH grant applications are required to be
submitted on time. Eventually, on time will mean
that a “clean” application (i.e., passed
Grants.gov and eRA Commons validations without
errors) has been submitted to Grants.gov by 5:00
p.m. local time on the receipt date. However, NIH
has provided some flexibility for the first few
submission dates of grant programs transitioning
to electronic submission. Applicant institutions
can submit changed/corrected applications in the
week (5 business days) following the submission deadline, provided
the changes made are needed to correct errors
encountered during the eRA business rule
validation process. This is not for tweaking the
content of a research plan, but for working
through specific errors on other fields of the
forms.NIH expects that all registration
requirements have been met prior to the initial
application submission and that the initial
application is submitted to Grants.gov on or
before the submission deadline. If the one-week
correction window is used, the application must
include a cover letter including the Grants.gov
tracking number for the original submission and an
explanation for why the corrected application is
required. The cover letter should be attached to
the PHS 398 Cover Letter form component found with
the optional documents in the application package.
 Back
to top Back
to top |
|
OPTIONS
FOR MACINTOSH®
USERS |
| Update (December 2006)—Special Edition Viewers for the Mac PowerPC and Intel processor-based Macs are now available on the Grants.gov
Download Software page. |
 Grants.gov has posted on their Web site an update
on IBM’s progress towards providing a PureEdge™
viewer compatible with Macs. They have included a
link to an early-release of an IBM Workplace Forms
(PureEdge) viewer for Macs on their
Download Software page and have identified
some of the limitations to this version of the
viewer (e.g., compatible only with PowerPC based
machines – G4 and G5). While this is not the
solution NIH and many of you had been hoping for,
it may be a viable option for some of you. We
strongly suggest that you read the available
documentation carefully before deciding whether
using the viewer is a good option for your
specific circumstances.
Grants.gov has posted on their Web site an update
on IBM’s progress towards providing a PureEdge™
viewer compatible with Macs. They have included a
link to an early-release of an IBM Workplace Forms
(PureEdge) viewer for Macs on their
Download Software page and have identified
some of the limitations to this version of the
viewer (e.g., compatible only with PowerPC based
machines – G4 and G5). While this is not the
solution NIH and many of you had been hoping for,
it may be a viable option for some of you. We
strongly suggest that you read the available
documentation carefully before deciding whether
using the viewer is a good option for your
specific circumstances.
As a reminder, Mac users can continue to use
the following proven options:
 |
NIH-hosted Citrix® servers: allow non-PC users to prepare and submit
applications using the PureEdge forms viewer. This service has been used
successfully by many applicants over the past year and has the capacity to
handle the anticipated load for the electronic submission of R01s in February
2007. |
 |
PC-emulation software: commercially available products allow Mac
users to run the PureEdge viewer. |
 |
Commercial Service Providers: offer a wide range of platform independent
services—from low-cost, single transaction options through full scale,
end-to-end grants management solutions. You should coordinate with your
institutions' grants office to explore these options further. |
We recognize fully that these options are an
interim solution, and that access to Grants.gov
PureEdge forms remains less than ideal for Mac
users. But, we have good news for the future.
Grants.gov plans to unveil a new
platform-independent solution based on Adobe forms
in April 2007. And NIH will migrate to the new
Adobe forms sometime between early summer and
October of 2007. Until then, applicants relying on
Grants.gov’s form-based solution will continue to
use the PureEdge viewer.
Information on further developments for Mac
users and contact information for
Grants.gov’s Customer Care Center may be found
on the
Grants.gov Web site.
 Back
to top Back
to top |
|
ELECTRONIC
SUBMISSION QUESTIONS: WHO, WHAT, WHERE |
|
 The
Electronic Submission Web site, the
application guide and Funding Opportunity
Announcements are great initial resources to
determine how to complete and submit electronic
applications. If you have checked these resources
and still need assistance, you can contact any one
of several support teams available to answer your
electronic application submission questions, as
indicated in the matrix below. The
Electronic Submission Web site, the
application guide and Funding Opportunity
Announcements are great initial resources to
determine how to complete and submit electronic
applications. If you have checked these resources
and still need assistance, you can contact any one
of several support teams available to answer your
electronic application submission questions, as
indicated in the matrix below.
TIP: Support desks can get busy during
heavy submission dates. Avoid phone delays by
taking advantage of online options like the eRA
Commons
Web support
ticket system.
 Back
to top Back
to top |
|
MULTIPLE
PI OPTION AVAILABLE BEGINNING FEBRUARY |
 Since
May 2006, the NIH has conducted a pilot involving
nine different Funding Opportunity Announcements
(FOAs) that attracted more than 60 applications
involving multiple Principal Investigators (PIs).
During the pilot, the NIH tested modifications to
electronic systems and operating policies
associated with grant applications involving more
than one PI. Upon interviews with a number of
applicants and peer reviewers, nearly all expressed
support for the concept and agreed that the
Multiple PI option would facilitate
interdisciplinary and other types of team
research. They also offered constructive comments
that have been incorporated into the instructions
and review criteria to be used in the future. Since
May 2006, the NIH has conducted a pilot involving
nine different Funding Opportunity Announcements
(FOAs) that attracted more than 60 applications
involving multiple Principal Investigators (PIs).
During the pilot, the NIH tested modifications to
electronic systems and operating policies
associated with grant applications involving more
than one PI. Upon interviews with a number of
applicants and peer reviewers, nearly all expressed
support for the concept and agreed that the
Multiple PI option would facilitate
interdisciplinary and other types of team
research. They also offered constructive comments
that have been incorporated into the instructions
and review criteria to be used in the future.
Reviewers raised some concerns about the
description of teamwork approaches included in
some of the applications. For example, in some
cases the listed PIs did not appear to have a
clearly identified leadership function or their
expertise did not appear to be closely related to
the proposed project. In all such cases, reviewers
pointed out that those issues detracted from the
perceived merit of the application. This type of
feedback has been vital towards clarifying the
instructions to applicants regarding the choice of
the multiple PI option.
Revised instructions to applicants and standard
review criteria to accommodate both single PI and
multiple PI applications will be built into both
the
PHS 398 and the SF424 (R&R) application packages as
well as FOAs that appear in the
NIH Guide for Grants and Contracts.
All relevant information will ultimately be
reflected and consolidated on the
NIH Multiple PI Web site.
The NIH has recently published the
Establishment
of Multiple Principal Investigator Awards for the
Support of Team Science Projects Guide
notice. To summarize the notice, most research grant
applications received electronically starting in
February 2007 will accommodate the Multiple PI
option. The decision to apply for a single PI or a
multiple PI grant will be the responsibility of
the investigators and the applicant organization.
Those decisions should be consistent with and
justified by the scientific goals of the project.
Salient features of the revised policy include:
 |
Applications have been modified to accommodate more than one PI (see
application forms). |
 |
Applications will include a Leadership Plan that describes the roles, the
responsibilities and the working relationship of the identified PIs. |
 |
All listed PIs will have access to Status on the
eRA Commons. |
 |
All PIs will be listed on summary statements. |
 |
All PIs will be listed on the Notice of Grant Award. |
 |
All PIs will be listed in
CRISP. |
 |
Awards involving PIs at different institutions will be managed using
subcontracts until options involving linked awards have been developed. |
 |
The role type "Co-PI" employed by other federal agencies will not be used by
NIH. |
NIH’s recognition of all leaders on a
team-managed project will encourage collaboration
among equals and multidisciplinary approaches when
that is the most appropriate way to address a
scientific problem.
 Back
to top Back
to top
|
|
MOST
POPULAR WEB PAGES ON THE ELECTRONIC SUBMISSION WEB
SITE |
 Since its debut in September 2005 and redesign
in May 2006, the
Electronic Submission of Grant
Applications Web site has been a valuable online
resource for people looking for information on NIH
and other Federal agencies’ electronic
grant application submission process using Grants.gov and the SF424
(R&R) forms package. Since its debut in September 2005 and redesign
in May 2006, the
Electronic Submission of Grant
Applications Web site has been a valuable online
resource for people looking for information on NIH
and other Federal agencies’ electronic
grant application submission process using Grants.gov and the SF424
(R&R) forms package.As traffic to the Web site averages 20,000 hits a
month, which pages are the most popular among
viewers? From September’s statistics for most
visited pages, the "Prepare Application" page wins
hands down.
As for the most downloaded file, the "NIH
Transition Plan," which details the dates each grant
program will transition to electronic submission,
is the clear winner.
Here are the top five most-trafficked Web pages for
the month of September:
|
1 |
Prepare Application
Contains the application guide, sample
applications, errors and warnings, a training video on the SF424 (R&R)
application, tips on completing an application. |
|
2 |
Prepare to Apply
Contains registration links for both Grants.gov
and eRA Commons, steps for registering in eRA Commons, a list of commercial
Service Providers, technical information for system-to-system developers. |
|
3 |
Avoiding Common Errors
Contains a list of the most common errors made by
applicants. This is a must-read to avoid having your application tripped by
errors. |
|
4 |
Frequently Asked Questions
Contains answers to the most frequently asked
questions about electronic submission. |
|
5 |
Timeline
A handy one pager that outlines NIH’s timeline for
transitioning grant programs from paper to electronic submission. |
When it comes to the most downloaded pages, these
five led the pack in September:
In addition, the following two resources provide basic information to
those new to the electronic grant application
submission process:
 Back
to top Back
to top |
|
NIH
GUIDE NOTICES |
|
NIH/AHRQ/NIOSH Confirm R01 Electronic Application Submission Plans for February 5 Receipt Date:
NIH/AHRQ/NIOSH will move forward with the R01
transition to electronic submission of grant
applications using the SF424 (R&R) forms for the
February 5, 2007 receipt date and beyond.
New Limits on Appendix Materials for All NIH/AHRQ/NIOSH Grant Applications Beginning with Receipt Dates
on or After January 3, 2007: Guidelines for the inclusion of
Appendix materials in grant applications have changed
to encourage applications to be as concise as possible
while containing the information needed for expert
scientific review. These changes take advantage of
electronic access to many publications and should make
application preparation and handling easier for both
applicants and reviewers.
Establishment
of Multiple Principal Investigator Awards for the
Support of Team Science Projects: Beginning
with research grant applications submitted for
February 2007 receipt dates, the NIH will allow
applicants and their institutions to identify more
than one Principal Investigator (PI). The Multiple PI
option will be extended to most research grant
applications submitted electronically through
Grants.gov using the SF424 R&R application package.
Announcing Electronic Grant Submission Training Opportunity: NIH
will hold a presentation on December 5, 2006,
to prepare the applicant community for the
upcoming transition of R01s to electronic submission.
Change in Standing Receipt Dates for NIH/AHRQ/NIOSH
Beginning in January 2007: New standing receipt
dates will be effective as of January 2007 and will
apply to both paper and electronic applications. The
new submission dates will allow for a steady flow of
applications rather than “boom and bust” cycles and
will maximize electronic system responsiveness.
Request for Information (RFI): Possible Page Limit Reduction
for the Research Plan Section of the Research Project Grant (R01) Application:
The NIH is exploring the possibility of reducing the
current 25 page limit for the Research Plan section of
the R01 application, as it has been suggested that NIH
peer review could be improved by focusing less on
experimental details and more on key ideas and the
scientific significance of proposed projects. Your
response to the RFI is greatly appreciated.
 Back
to top Back
to top |
|
FEEDBACK |
|
Feedback from recipients and
subscribers of the NIH Extramural Nexus is
vital. Comments, questions, and suggestions for
topics will enable Nexus editorial staff to
deliver appropriate content to the grantee
community.
 Back
to top Back
to top |
|
PRINT
VERSION |
|
This
issue's printer-friendly version
(Adobe® Reader® Required)
 Back
to top Back
to top |
|
|
|
  |
|
  |
|
|
The
NIH Extramural Nexus is a bimonthly update
from the NIH Office of Extramural Research. Send articles,
comments, questions and suggestions to
the Editor.
The
NIH Extramural Nexus reserves the right to
select and edit submissions. To subscribe to the NIH Extramural Nexus, send a
plain text email to Listserv@list.nih.gov
including only the words Subscribe EXTRAMURALNEXUS
in the body of the message. To unsubscribe, follow the
same procedure, using the words Unsubscribe
EXTRAMURALNEXUS in the message body.
|
|
|
NIH
Extramural Nexus Web site and archives |
|
|
