
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-393 - Sodium Fluoride
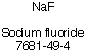
| Chemical Formula: NaF |
|---|
Sodium fluoride is a white, crystalline, water-solublepowder used in municipal water fluoridation systems, in various dentalproducts, and in a variety of industrial applications. Toxicology andcarcinogenesis studies were conducted with F344/N rats and B6C3F1 mice ofeachsex by incorporating sodium fluoride into the drinking water in studies lasting14 days, 6 months, and 2 years. In addition, genetic toxicology studies wereperformed with Salmonella typhimurium, with mouse L5178Y cells, andwithChinese hamster ovary cells.
Rats and mice received sodium fluoride in drinking water atconcentrations as high as 800 ppm. (Concentrations are expressed as sodiumfluoride; fluoride ion is 45% of the sodium salt by weight.) In the high-dosegroups, 5/5 male and 5/5 female rats and 2/5 male mice died; one female ratwasgiven 400 ppm in the drinking water also died before the end of the studies.No gross lesions were attributed to sodium fluoride administration.
Rats received concentrations of sodium fluoride in drinking water ashigh as 300 ppm, and mice as high as 600 ppm. No rats died during thestudies;however, among the mice, 4/9 high-dosemales, 9/11 high-dosefemales, and 1/8 males in the 300 ppm group died before the end of thestudies.Weight gains were less than those of controls for rats receiving 300 ppm andmice receiving 200 to 600 ppm.
The teeth of rats and mice receiving the higher doses of sodium fluoride werechalky white and chipped or showed unusual wear patterns. Mice and maleratsgiven the higher concentrations had microscopic focal degeneration of theenamel organ. Rats receiving 100 or 300 ppm sodium fluoride had minimalhyperplasia of the gastric mucosa of the stomach, and one high-doserat of each sex had an ulcer. Acute nephrosis and/or lesions in the liver andmyocardium were observed in mice that died early, and minimal alterations inbone growth/remodeling were observed in the long bones of mice receivingsodiumfluoride at concentrations of 50 to 600 ppm.
The sodium fluoride concentrations selected for the 2-yearstudies in both rats and mice were 0, 25, 100, and 175 ppm in the drinkingwater. These concentrations were selected based on the decreased weightgainof rats at 300 ppm and of mice at 200 ppm and above, on the incidence ofgastric lesions in rats at 300 ppm in the 6-monthstudies, and on the absence of significant toxic effects at sodiumfluoride concentrations as high as 100 ppm in an earlier 2-yearstudy.
Mean body weights of dosed and control groups of rats and miceweresimilar throughout the 2-yearstudies. Survival of rats and mice was not affected by sodium fluorideadministration. Survival rates after 2 years were: male rats-control, 42/80;25 ppm, 25/51; 100 ppm, 23/50; 175 ppm, 42/80; female rats-59/80;31/50; 34/50; 54/81; male mice-58/79; 39/50; 37/51; 65/80; female mice-53/80;38/52; 34/50; 52/80.
The teeth of rats and mice has a dose-dependentwhitish discoloration, and male rats had an increased incidence of toothdeformities and attrition leading on occasion to malocclusion. The teeth ofmale and, to a lesser degree, female rats had areas of microscopic dentinedysplasia and degeneration of ameloblasts. Dentine dysplasia occurred inbothdosed and control groups of male and female mice; the incidence of this lesionwas significantly greater in high-dosethan in control male mice. Osteosclerosis of long bones was increased infemale rats given drinking water containing 175 ppm sodium fluoride. Noothersignificant nonneoplastic lesions in rats or mice appeared related to sodiumfluoride administration.
Osteosarcomas of bone were observed in 1/50 male rats in the 100 ppmgroup andin 3/80 male rats in the 175 ppm group. None were seen in the control or 25ppm dose groups. One other 175 ppm male rat had an extraskeletalosteosarcomaarising in the subcutaneous tissue. Osteosarcomas occur in historical controlmale rats at an incidence of 0.5% (range 0-6%). The historical incidence is not directly comparable with the incidencesobserved in this study because examination of bone was morecomprehensive inthe sodium fluoride studies than in previous NTP studies of other chemicals,and the diet used in previous studies was not controlled for fluoride content.In the current study, although the pairwise comparison of the incidence in the175 ppm group versus that in the controls was not statistically significant,osteosarcomas occurred with a statistically significant dose-responsetrend, leading to the conclusion that a weak association may exist between theoccurrence of these neoplasms and the administration of sodium fluoride. Noother neoplastic lesions in rats or mice were considered possibly related tochemical administration.
Sodium fluoride was negative for gene mutationinductionin Salmonella typhimurium strains TA100, TA1535, TA1537, and TA98withand without S9. In two laboratories, sodium fluoride was tested for inductionof trifluorothymidine resistance in mouse L5178Y lymphoma cells; resultswerepositive both with and without S9. Sodium fluoride was tested for cytogeneticeffects in Chinese hamster ovary (CHO) cells in two laboratories. In the firstlaboratory, the sister chromatid exchange (SCE) test was negative with andwithout S9, and the chromosomal aberration (Abs) test was positive in theabsence of S9; in the second laboratory, the SCE test was positive with andwithout S9, but no induction of Abs was observed. The laboratory thatreporteda negative result for Abs tested at doses below that shown to be positive atthe other laboratory. Similarly, the positive SCE result was obtained at ahigher dose and longer harvest time than used by the laboratory reporting thenegative SCE response.
Under the conditions of these 2-yeardosed water studies, there was equivocal evidence of carcinogenicactivity of sodium fluoride in male F344/N rats, based on the occurrence ofa small number of osteosarcomas in dosed animals. "Equivocal evidence" is acategory for uncertain findings defined as studies that are interpreted asshowing a marginal increase of neoplasms that may be related to chemicaladministration. There was no evidence of carcinogenic activity infemale F344/N rats receiving sodium fluoride at concentrations of 25, 100, or175 ppm (11, 45, or 79 ppm fluoride) in drinking water for 2 years. There wasno evidence of carcinogenic activity of sodium fluoride in male orfemale mice receiving sodium fluoride at concentrations of 25, 100, or 175ppmin drinking water for 2 years.
Dosed rats had lesions typical of fluorosis of the teeth and female ratsreceiving drinking water containing 175 ppm sodium fluoride had increasedosteosclerosis of long bones.
Report Date: December 1990
Pathology Tables, Survival and Growth Curves from NTP 2-year Studies
Target Organs & Incidences from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on May 05, 2006
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.