
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-333 - N-Phenyl-2-Naphthylamine
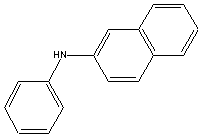
| Chemical Formula: C16H13N | - | 3D Structure* |
|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | ||
N-Phenyl- 2- naphthylamine, formerly used as a antioxidant in the rubber industry, was selected for toxicology and carcinogenesis studies because at the time of nomination (1976) it had a large annual production and widespread human exposure. Additional reasons for selection included it structural similarity and possible metabolism to the known human urinary bladder carcinogen, 2-naphthylamine. Toxicology and carcinogenesis studies were conducted by feeding diets containing N-phenyl-2-naphthylamine (approximately 98% pure and containing less than 1 ppm 2-naphthylamine) at various concentrations to groups of F344/N rats and B6C3F1 mice of each sex for 14 days, 13 weeks, or 2 years.
In 14-day studies, 3/5 male and 4/5 female rats that received 50,000 ppm N-phenyl-2-naphthylamine died before the end of the studies. Final mean body weights of rats that received 12,500 ppm or more were considerably lower (18%-57%) than those of the controls. Arched backs, rough coats, and diarrhea were observed for males that received 12,500 ppm or more and for females that received 25,000 or 50,000 ppm. All mice were alive at the end of the studies, and no compound-related clinical signs of toxicity were observed in mice given feed containing up to 20,000 ppm.
In 13-week studies, deaths occurred in 4/10 male and 9/10 female rats that received the highest dose (40,000 ppm) of N-phenyl-2-naphthylamine. Final mean body weights of rats that received 5,000-40,000 ppm were 9%-60% lower than those of the controls. The liver weight to body weight ratios increased with increasing dose, with the ratios for male rats at 10,000 ppm or more and for female rats at 5,000 ppm being greater (P<0.05) than those of controls. A compound-related nephropathy occurred in rats and was characterized by renal tubular epithelial degeneration and hyperplasia. Other effects in rats included hematopoietic hypoplasia or atrophy of the femoral bone marrow, testicular hypospermatogenesis, lymphoid degeneration of the thymus, and lymphoid depletion of the spleen.
In mice, 2/10 males and 7/10 females that received 40,000 ppm died before the end of the 13-week studies. The final mean body weights of mice that received 10,000, 20,000, or 40,000 ppm were 9%-32% lower than those of the controls. The liver weight to body weight ratios for mice increased with increasing dose. Those for male mice at 10,000 ppm or more and for female mice at 20,000 ppm or more were greater (P<0.05) than those for the controls. Nephropathywas observed at increased incidences and severity in dosed mice.
Because of kidney lesions, liver enlargement, lower weight gain, and increased mortality in the shorter term studies, dietary concentrations of N-phenyl-2-naphthylamine selected for the 2-year studies in rats and mice were 0, 2,5000, and 5,000 ppm.
The mean body weights of dosed rats were lower than those of the controls throughout the studies (12% and 16% lower for dosed males and 15% and 31% lower for dosed females at the end of the studies). The average daily feed consumption for rats was 94%-87% that of the controls for dosed males and 88% that of the controls for dosed females. The estimated average amount of N-phenyl-2-naphthylamine consumed per day was 100 mg/kg and 225 mg/kg for male rats and 120 mg/kg and 260 mg/kg for female rats. The survival of the high dose group of male rats was greater (P<0.05) than that of the controls after week 101 (male: control, 24/50; low dose, 28/50; high dose, 34/50; female: 26/50; 44/50; 38/50).
Final mean body weights of high dose male and female mice were lower (male, 9%; female, 23%) than those of the controls. The estimated average daily feed consumption by dosed mice was within 10% that of the controls. The average amount of N-phenyl-2-naphthylamine consumed per day was approximately 500 or 1,000 mg/kg for male mice and 450 or 900 mg/kg for female mice. No significant differences in survival were observed between any groups of mice of either sex (male: control, 33/50; low dose, 36/50; high dose, 28/50; female: 36/50; 30/50; 35/50).
As in the 13-week studies, the kidney was the principal target for toxic effects of N-phenyl-2-naphthylamine. Mineralization of the kidney, necrosis of the renal papilla, and epithelial hyperplasia and calculi of the kidney pelvis were observed at increased incidences in high dose female rats. Hydronephrosis, atrophy, fibrosis, and chronic focal inflammation of the kidney were observed at increased incidences in high dose female rats. Cysts and acute suppurative inflammation of the kidney were observed at increased incidences in dosed male and high dose female rats. No compound-related renal neoplasms were observed in rats.
Nuclear enlargement of renal tubular epithelial cells and nephropathy were observed at increased incidences in high dose female mice. Atypical tubular cell hyperplasia occurred in two high dose female mice. A tubular cell adenoma was found in one high dose female mouse, and a tubular cell adenocarcinoma was found in another high dose female mouse. No renal neoplasms were observed in dosed male mice.
Neoplasms of several organs occurred in rats with negative trends and/or at significantly lower incidences in high dose groups. These included thyroid gland C-cell neoplasms in males and females and mammary gland fibroadenomas, pituitary gland adenomas, and mononuclear cell leukemia in females. The lack of carcinogenicity in rats may be related to an inability to metabolize this compound to the known animal and human carcinogen 2-napththylamine.
N-Phenyl-2-naphthylamine was not mutagenic in the Salmonella typhimurium/microsome assay with strains TA97, TA98, TA100, or TA1535 with or without induced hamster or rat liver S9. The chemical did not induce chromosomal aberrations in cultured Chinese hamster ovary (CHO) cells with or without metabolic activation. No increase in sister chromatid exchanges (SCEs) was observed in the absence of metabolic activation; in the presence of rat liver S9, the SCE results were judged to be equivocal.
The data, documents, and pathology materials from the 2-year studies of N-phenyl-2-naphthylamine were audited at the NTP Archives. The audit findings show thatthe conduct of the studies is documented adequately and support the data and results given in this Technical Report.
Under the conditions of these 2-year feed studies, there was no evidence of carcinogenic activity for male or female F344/N rats fed diets containing 2,500 or 5,000 ppm N-phenyl-2-naphthylamine. Decreased incidences of several neoplasms were observed in dosed rats: thyroid gland C-cell neoplasms in males and females and mononuclear cell leukemia, pituitary gland adenomas, and mammary gland fibroadenomas in females. There was no evidence of carcinogenic activity for male B6C3F1 mice fed diets containing 2,500 or 5,000 ppm N-phenyl-2-naphthylamine. There was equivocal evidence of carcinogenic activity of N-phenyl-2-naphthylamine for female B6C3F1 mice as indicated by the occurrence of two rare kidney neoplasms. Chemical-related nonneoplastic lesions (nephropathy, karyomegaly, and hyperplasia) occurred in the kidney of rats and mice.
Synonyms: N-(2-naphthyl)aniline; 2-naphthylphenylamine; b-naphthylphenylamine; 2-phenylaminonaphthalene; phenyl-b-naphthylamine; N-phenyl-b-naphthylamine
Trade Names: Aceto PBN; Agerite Powder: Antioxidant 116; Neosone D; Neozon D; Nilox PBNA; Nonox D; PBNA; Stabilizator AR
Report Date: January 1988
Target Organs & Incidences from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 14, 2004
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.