
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-478 - Diethanolamine
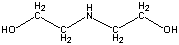
| Chemical Formula: C4H11NO2 | - | 3D Structure* |
|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | ||
Diethanolamine is widely used in the preparation of diethanolamides and diethanolamine salts of long-chain fatty acids that are formulated into soaps and surfactants used in liquid laundry and dishwashing detergents, cosmetics, shampoos, and hair conditioners. Diethanolamine is also used in textile processing, in industrial gas purification to remove acid gases, as an anticorrosion agent in metalworking fluids, and in preparations of agricultural chemicals. Aqueous diethanolamine solutions are used as solvents for numerous drugs that are administered intravenously. Diethanolamine was selected for evaluation because its large-scale production and pattern of use indicate the potential for widespread human exposure. Male and female F344/N rats and B6C3F1 mice received dermal applications of diethanolamine in 95% ethanol for 2 years. Genetic toxicology studies were performed in Salmonella typhimurium, L5178Y mouse lymphoma cells, cultured Chinese hamster ovary cells, and B6C3F1 mouse peripheral blood erythrocytes.
Groups of 50 male rats were administered 0, 16, 32, or 64 mg diethanolamine/kg body weight in ethanol dermally for 2 years. Groups of 50 female rats were administered 0, 8, 16, or 32 mg/kg in ethanol dermally for 2 years.
Survival, Body Weights, and Clinical Findings
Survival of vehicle control and dosed male and female rats was similar. Mean body weights of 64 mg/kg males were less than those of the vehicle controls beginning week 8, and mean body weights of females were generally similar to those of the vehicle control group. The only clinical finding attributed to diethanolamine administration was irritation of the skin at the site of application.
Pathology Findings
Minimal to mild nonneoplastic lesions occurred at the site of application in the epidermis of dosed male and female rats. The incidence of acanthosis in 64 mg/kg males, the incidences of hyperkeratosis in 32 and 64 mg/kg males and in all dosed female groups, and the incidences of exudate in 64 mg/kg males and in all dosed female groups were greater than those in the controls.
The incidences and severities of nephropathy were significantly increased in dosed female rats compared to the vehicle controls.
Groups of 50 male and 50 female mice were administered 0, 40, 80, or 160 mg diethanolamine/kg body weight in ethanol dermally for 2 years.
Survival, Body Weights, and Clinical Findings
Survival of dosed male groups was similar to that of the vehicle control group; survival of dosed female groups was significantly less than that of the vehicle control group. Mean body weights of 80 and 160 mg/kg males were less than those of the vehicle controls after weeks 88 and 77, respectively. Mean body weights of dosed groups of females were generally less than those of the vehicle controls during the second year of the study.
Pathology Findings
In male mice, the incidences of hepatocellular adenoma and of hepatocellular adenoma or carcinoma (combined) in all dosed groups and of hepatocellular carcinoma and hepatoblastoma in 80 and 160 mg/kg males were significantly increased compared to the vehicle controls. The incidences of hepatocellular neoplasms were significantly greater in dosed groups of female mice than in the vehicle control group. The incidences of hepatocellular neoplasms in all dosed groups of males and females exceeded the historical control ranges. Nonneoplastic hepatocyte changes were seen only in dosed male and female mice. Changes consisted of cytoplasmic alteration and syncytial alteration.
The incidences of renal tubule adenoma in males occurred with a positive trend; however, the incidences of carcinoma and hyperplasia did not follow this pattern. An extended evaluation of kidney step sections revealed additional adenomas and hyperplasias in all dosed groups. The combined analysis of single and step sections indicated a dose-related increase in the incidences of renal tubule hyperplasia and renal tubule adenoma or carcinoma (combined), and an increase in the incidences of renal tubule adenoma in male mice.
Incidences of thyroid gland follicular cell hyperplasia were increased in dosed male and female mice compared to vehicle controls.
Hyperkeratosis, acanthosis, and exudate were treatment-related changes in the skin at the site of application. The incidences of hyperkeratosis were significantly greater than those in the vehicle control groups in all dosed groups except 40 mg/kg females.
Diethanolamine was not mutagenic in any of four strains of Salmonella typhimurium, in the presence or absence of S9 metabolic activation enzymes. No induction of trifluorothymidine resistance was observed in L5178Y mouse lymphoma cells treated with diethanolamine with or without S9. Diethanolamine did not induce significant sister chromatid exchanges or chromosomal aberrations in cultured Chinese hamster ovary cells, with or without S9. Peripheral blood samples collected from male and female mice exposed to 80 to 1,250 mg/kg diethanolamine dermally for 13 weeks showed no increase in micronucleated normochromatic erythrocytes.
Under the conditions of these 2-year dermal studies, there was no evidence of carcinogenic activity of diethanolamine in male F344/N rats administered 16, 32, or 64 mg/kg diethanolamine or in female F344/N rats administered 8, 16, or 32 mg/kg. There was clear evidence of carcinogenic activity of diethanolamine in male and female B6C3F1 mice based on increased incidences of liver neoplasms in males and females and increased incidences of renal tubule neoplasms in males.
Dermal administration of diethanolamine to rats was associated with increased incidences of acanthosis (males only), hyperkeratosis, and exudate of the skin and increased incidences and severities of nephropathy in females. Dermal administration of diethanolamine to mice was associated with increased incidences of cytoplasmic alteration (males only) and syncytial alteration of the liver, renal tubule hyperplasia (males only), thyroid gland follicular cell hyperplasia, and hyperkeratosis of the skin.
Synonyms: Bis-2-hydroxyethylamine; DEA, diethylolamine; 2,2'-dihydroxydiethylamine; diolamine; 2,2'-iminobisethanol; 2,2'-iminodiethanol; iminodiethanol
Summary of the 2-Year Carcinogenesis and Genetic Toxicology Studies of Diethanolamine
|
|
Male F344/N Rats |
Female F344/N Rats |
Male B6C3F1 Mice |
Female B6C3F1 Mice |
|---|---|---|---|---|
| Doses in ethanol by dermal application |
0, 16, 32, or 64 mg/kg |
0, 8, 16, or 32 mg/kg |
0, 40, 80, or 160 mg/kg |
0, 40, 80, or 160 mg/kg |
| Body weights |
64 mg/kg groups generally less than vehicle control groups |
Dosed groups generally similar to vehicle control group |
80 and 160 mg/kg groups less than vehicle control group |
Dosed groups generally less than vehicle control group |
| Survival rates |
14/50, 10/50, 21/50, 22/50 |
25/50, 29/50, 29/50, 24/50 |
40/50, 43/50, 34/50, 30/50 |
44/50, 33/50, 33/50, 23/50 |
| Nonneoplastic effects |
Skin: acanthosis (0/50, 2/50, 4/50, 10/50); hyperkeratosis (0/50, 3/50, 5/50, 11/50); exudate (0/50, 3/50, 2/50, 7/50)
|
Skin: hyperkeratosis (3/50, 13/50, 23/50, 23/50); exudate (1/50, 7/50, 7/50, 7/50)
Kidney: nephropathy (40/50, 47/50, 48/50, 48/50); severity (1.2, 1.5, 1.9, 2.7) |
Liver: cytoplasmic alteration (1/50, 17/50, 17/50, 12/50); syncytial alteration (0/50, 28/50, 38/50, 23/50)
Kidney: renal tubule hyperplasia (standard and extended evaluation combined (3/50, 7/50, 7/50, 10/50) Thyroid gland: follicular cell hyperplasia (18/50, 22/49, 30/50, 42/50) Skin: hyperkeratosis (0/50, 13/50, 10/50, 17/50) |
Liver: syncytial alteration (0/50, 2/50, 17/50, 18/50)
Thyroid gland: follicular cell hyperplasia (18/50, 28/49, 32/50, 39/50) Skin: hyperkeratosis (1/50, 3/50, 8/50, 16/50) |
| Neoplastic effects |
None |
None |
Liver: hepatocellular adenoma (31/50, 42/50, 49/50, 45/50); hepatocellular carcinoma (12/50, 17/50, 33/50, 34/50); hepatoblastoma (0/50, 2/50, 8/50, 5/50); hepatocellular adenoma, hepatocellular carcinoma, or hepatoblastoma (39/50, 47/50, 50/50, 49/50)
Kidney: adenoma (standard evaluation -1/50, 4/50, 6/50, 6/50; standard and extended evaluation combined - 1/50, 6/50, 8/50, 7/50); adenoma or carcinoma (combined) (standard evaluation -3/50, 5/50, 6/50, 8/50; standard and extended evaluation combined - 3/50, 7/50, 8/50, 9/50) |
Liver: hepatocellular adenoma (32/50, 50/50, 48/50, 48/50); hepatocellular carcinoma (5/50, 19/50, 38/50, 42/50); hepatocellular adenoma or carcinoma (33/50, 50/50, 50/50, 50/50) |
| Level of evidence of carcinogenic activity |
No evidence |
No evidence |
Clear evidence |
Clear evidence |
| Genetic toxicology | |||||
|---|---|---|---|---|---|
| Salmonella typhimurium gene mutations: | Negative with and without S9 in strains TA98, TA100, TA1535, and TA1537 | ||||
|
Mouse lymphoma gene mutations: |
Negative |
||||
|
Sister chromatid exchanges Cultured Chinese hamster ovary cells in vitro: |
Negative |
||||
|
Chromosomal aberrations Cultured Chinese hamster ovary cells in vitro: |
Negative |
||||
|
Micronucleated erythrocytes
Mouse peripheral blood in vivo: |
Negative |
||||
Report Date: July 1999
Pathology Tables, Survival and Growth Curves from NTP 2-year Studies
Target Organs and Incidences for 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 11, 2007
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.