
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-251 - 2,4- & 2,6-Toluene Diisocyanate
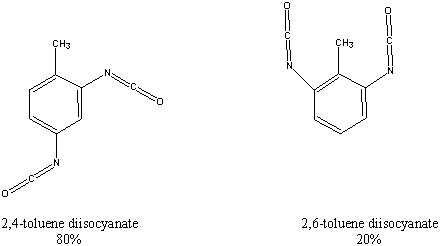
| Chemical Formula: C9H6N2O2 | - | 3D Structure* |
|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | ||
Toluene diisocyanate (TDI) is commercially produced as an approximate 80:20 mixture of the 2,4- and 2,6-isomers. In 1980, 580,000 pounds of this chemical were produced in the United States, primarily for use in the manufacture of flexible polyurethane foams. These foam elastomers are found in furniture and automobile cushions, carpet underlays, pillow filling, mattresses, insulation, shoes, purses, and toys. TDI is also used to produce polyurethane coatings for lacquers and wood finishes.
Groups of 50 female F344/N rats and 50 B6C3F1 mice were administered commercial grade toluene diisocyanate (80% 2,4- and 20% 2,6-) in corn oil by gavage at doses of 60 or 120 mg/kg body weight, 5 days per week for 105 or 106 weeks. Groups of 50 male F344/N rats received 30 or 60 mg/kg and groups of 50 male B6C3F1 mice received 120 or 240 mg/kg on the same schedule. Dosage analyses of toluene diisocyanate indicated that the chemical had reacted in the corn oil vehicle, resulting in actual gavage concentrations 77% to 90% of theoretical values. Groups of 50 rats and 50 mice of each sex received corn oil only and served as vehicle controls.
Survival in all groups of dosed rats in the 2-year studies were shorter (P<0.005) than that of the controls; depressions of the mean body weight gain relative to controls were greater than 10% in all dosed rat groups throughout most of the study. A dose-dependent pattern of cumulative toxicity began at 70 weeks and culminated in excessive mortality, indicating the estimated tolerated dose had been exceeded for rats. Acute bronchopneumonia occurred at increased incidences in groups of dosed male and female rats (males: control, 2/50; low dose, 6/50; high dose, 14/50; females: 1/50, 10/50, 25/49).
Subcutaneous tissue fibromas or fibrosarcomas (combined) in male rats occurred with a positive trend (P<0.01; 3/50, 6/50, 12/50). The incidence in the high dose group was higher than that in the controls (P<0.01). The same tumor comparisons were significant (P<0.001) in female rats by the life table analysis. Mammary gland fibroadenomas in female rats occurred with a positive trend (P<0.001), and the incidences in low and high dose groups were significantly higher than that in controls (P<0.01).
Pancreatic acinar cell adenomas in male rats occurred with a positive trend (P<0.05; 1/47, 3/47, 7/49). The incidence in the high dose group was higher than that in the controls (P<0.05).
The incidences of pancreatic islet cell adenomas in female rats were higher by the incidental tumor test (P<0.01) in low dose (6/49) and high dose (2/47) groups than in controls (0/50). An islet cell carcinoma was also observed in a low dose female rat. The incidences of female rats with neoplastic nodules in the liver occurred with a positive trend (P<0.05; 3/50, 8/50, 8/48), and the incidence in the high dose group was higher (P<0.05) than that in the controls.
Survival of high dose male mice in the 2-year study was significantly shorter than that of the controls (P<0.001). During the second year of the study, mean body weight gains of high dose male mice were less than those of the controls. Cytomegaly of kidney tubular epithelium was found in 45/48 (94%) low dose male mice and 41/50 (82%) high dose male mice but not in any of the controls.
Hemangiomas or hemangiosarcomas (combined) of the circulatory system in female mice occurred with a positive trend (P<0.01; control, 0/50; low dose, 1/50; high dose, 5/50). The incidence in the high dose group was significantly higher than that in the controls (P<0.05).
Hepatocellular adenomas in female mice occurred with a positive trend (P<0.001; 2/50, 3/50, 12/50), and the incidence in the high dose group was higher than that in the controls (P<0.01).
Toluene diisocyanate was mutagenic in Salmonella typhimurium strains TA98 and TA100 in the presence (but not the absence) of Aroclor 1254-induced male Sprague-Dawley rat or male Syrian hamster liver S9; it was not mutagenic in strains TA 1535 or 1537.
An audit of the experimental data for these 2-year toxicological and carcinogenicity studies on commercial grade 2,4- and 2,6-toluene diisocyanate was conducted. There were no data discrepancies that influenced the final interpretations.
Under the conditions of these gavage studies, commercial grade toluene diisocyanate in corn oil was carcinogenic for F344/N rats, causing subcutaneous fibromas and fibrosarcomas (combined) in males and females, pancreatic acinar cell adenomas in males, and pancreatic islet cell adenomas, neoplastic nodules of the liver, and mammary gland fibroadenomas in females. Toluene diisocyanate was not carcinogenic for male B6C3F1 mice. TDI was carcinogenic for female B6C3F1 mice, causing hemangiomas or hemangiosarcomas (combined), as well as hepatocellular adenomas.
Levels of Evidence of Carcinogenicity: | ||
| Male Rats: | Positive | |
| Female Rats: | Positive | |
| Male Mice: | Negative | |
| Female Mice: | Positive | |
Synonym: TDI
Report Date: August 1986
Target Organs & Incidences from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 14, 2004
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.