
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-243 - Trichloroethylene
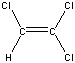
| Chemical Formula: C2HCl3 | - | 3D Structure* |
|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | ||
Trichloroethylene (TCE) is an industrial solvent used for vapor degreasing and cold cleaning of fabricated metal parts. TCE has also been used as a carrier solvent for the active ingredients of insecticides and fungicides, as a solvent for waxes, fats, resins, and oils, as an anesthetic for medical and dental use, and as an extractant for spice oleoresins and for caffeine from coffee. Trichloroethylene may be found in printing inks, varnishes, adhesives, paints, lacquers, spot removers, rug cleaners, disinfectants, and cosmetic cleansing fluids. TCE may also be used as a chain terminator in polyvinyl chloride production and as an intermediate in the production of pentachloroethane. Trichloroethylene is no longer used with food, drugs, or cosmetics.
Carcinogenesis studies of epichlorohydrin-free trichloroethylene were conducted by administering the test chemical in corn oil by gavage to groups of 50 male and 50 female F344/N rats and B6C3F1 mice. Dosage levels were 500 and 1,000 mg/kg for rats and 1,000 mg/kg for mice. Trichloroethylene was administered five times per week for 103 weeks, and surviving animals were killed between weeks 103 and 107. Groups of 50 rats and 50 mice of each sex received corn oil by gavage on the same schedule and served as vehicle controls. Groups of 50 male and 50 female rats were used as untreated controls.
The dosage levels selected for the 2-year study were based on the results of the 13-week studies. Groups of 10 male and 10 female rats received TCE by gavage at doses of 125 to 2,000 mg/kg (males) and 62.5 to 1,000 mg/kg (females) for 13 weeks. Groups of 10 male and 10 female mice received gavage doses of 375 to 6,000 mg/kg of TCE for 13 weeks. Survival, body weight gains, and previous experience with TCE were used to select doses for the 2-year study. All rats survived the 13-week study, but males receiving 2,000 mg/kg exhibited a 24% difference in final body weight. At the 1,000 mg/kg dose, final body weights for males (-3%) and for females (-2%) were similar to those of controls. The doses selected for the 2-year study in rats were 500 and 1,000 mg/kg for both sexes. The initial doses used in the earlier bioassay in Osborne-Mendel rats were 549 and 1,097 mg/kg for both sexes. A total of 8/10 male mice and 10/10 female mice receiving doses of TCE as high as 1,500 mg/kg survived the 13-week experimental period. The single dosage level selected for the 2-year study in mice was 1,000 mg/kg for both sexes. This dose was less than the high dose used in the earlier bioassay in B6C3F1 mice (2,339 mg/kg for males and 1,739 for females) and was similar to the previous low doses (1,169 mg/kg for males and 869 for females).
In the 2-year study, the survival of both low and high dose male rats and dosed male mice was less (P<0.005) than that of the vehicle controls. Mean body weights of dosed rats of each sex were lower than those of the vehicle controls, and after week 65, the decrements in body weight gains were dose related. The mean body weight of dosed male mice was lower than that of the vehicle controls throughout the study, while those of the dosed and vehicle control female mice were comparable.
Cytomegaly (toxic nephrosis) of the kidney was observed in 96/98 male and in 97/97 female rats given TCE, with none being found in male or female vehicle control rats. This lesion was more severe in males, particularly in the high dose group. Cytomegaly was observed in 45/50 male mice and in 48/49 female mice administered TCE, and in none of the vehicle controls. Renal tubular cell adenocarcinomas were found in the three high dose male rats; these neoplasms were observed in those male rats killed at the end of the study (0/33, 0/20, and 3/16, 19%). The incidence in the high dose male rats at the end of the study was greater (P<0.05) than that in the controls. Renal tubular cell adenocarcinomas are considered uncommon occurrences in F344/N rats, with 3/748 (0.4%) being observed in historical vehicle gavage controls. Additional renal tumors in dosed male rats included one transitional cell carcinoma of the renal pelvis and two tubular cell adenomas in low dose animals and one carcinoma of the renal pelvis in a high dose animal. No renal neoplasms were found in vehicle control rats; one untreated control male rat had a transitional cell papilloma of the renal pelvis. In female rats, one tubular cell adenocarcinoma was found in the high dose group.
An increased incidence (P<0.05, life table) of peritoneal mesotheliomas was detected in low dose male rats (control, 1/50; low dose, 5/50; high dose, 1/49). Mesotheliomas have been diagnosed in 16/752 (2.1%) historical vehicle control male F344/N rats, and the increased incidence in the present study may have been related to the administration of TCE.
The results in male F344/N rats were considered equivocal for detecting a carcinogenic response because both groups receiving TCE showed significantly reduced survival compared to vehicle controls (35/50, 70%, 20/50, 40%; 16/50, 32%) and because 20% of the animals in the high dose group were killed accidently by gavage error.
Negative trends were observed for chromophobe adenomas of the pituitary gland and for endometrial stomal polyps in female rats. These decreases were not considered to be related to the administration of TCE.
The administration of TCE to mice caused increased incidences of hepatocellular carcinoma in males (control, 8/48; dosed, 31/50; P<0.001) and in females (control, 2/48; dosed, 13/49; P<0.005). Hepatocellular carcinomas metastasized to the lungs in five dosed male mice and one control male mouse, and none were observed in females. The incidence of hepatocellular adenomas was increased in male mice (control, 7/48; dosed 14/50) and in female mice (control, 4/48; dosed, 16/49; P<0.05).
Under the conditions of these studies, epichlorohydrin-free trichloroethylene caused renal tubular-cell neoplasms in male F344/N rats, produced toxic nephrosis in both sexes, and shortened the survival time of males. This experiment in male F344/N rats was considered to be inadequate to evaluate the presence or absence of a carcinogenic response to trichloroethylene. For female F344/N rats receiving trichloroethylene, containing no epichlorohydrin, there was no evidence of carcinogenicity. Trichloroethylene (without epichlorohydrin) was carcinogenic for B6C3F1 mice, causing increased incidences of hepatocellular carcinomas in males and females and of hepatocellular adenomas in females.
Levels of Evidence of Carcinogenicity: | ||
| Male Rats: | Inadequate Study | |
| Female Rats: | Negative | |
| Male Mice: | Positive | |
| Female Mice: | Positive | |
Synonym: TCE
Note: Trichloroethylene was previously tested in Osborne-Mendel rats and B6C3F1 mice by gavage (See TR-2, reported 1977) and also in four rat strains (ACI, August, Marshall, and Osborne-Mendel) by gavage (See TR-273, reported 1988).
Report Date: May 1990
Target Organs & Incidences from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 14, 2004
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.