Appendix B: Mouse Embryonic Stem Cell Cultures
The techniques for culturing mouse embryonic stem (ES) cells from the inner cell mass of the preimplantation blastocyst were first reported 20 years ago [6, 11], and versions of these standard procedures are used today in laboratories throughout the world. Additionally, studies of embryonal carcinoma (EC) cells from mice and humans [2, 30] have helped establish parameters for growing and assessing ES cells. It is striking that, to date, only three species of mammals have yielded long-term cultures of self-renewing ES cells: mice, monkeys, and humans [21, 34, 35, 36] (see Figure B.1. Origins of Mouse Pluripotent Stem Cells).
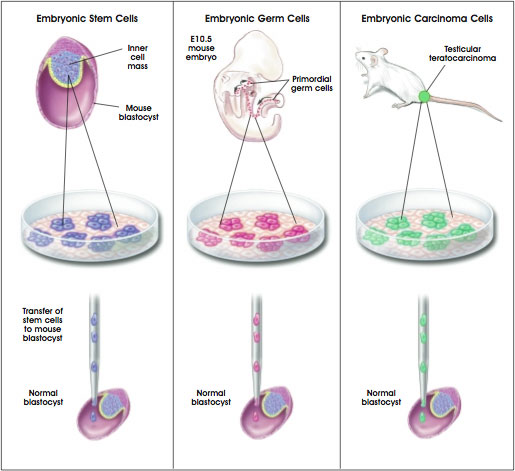
Figure B.1. Origins of Mouse Pluripotent Stem Cells.
(© 2001 Terese Winslow)
In mice, the efficiency of generating ES cells is influenced by the genetic strain of laboratory mice and individual factors that affect pregnant females. Only a few strains of laboratory mice—notably 129, C57BL/6, and a hybrid strain—yield cultures of ES cells. Even then, ES cells derived from C57BL/6 blastocysts do not behave as reliably as do ES cells from the 129 strain of mice. The former are more difficult to propagate in vitro, generate chimeras less efficiently than do ES cells from the 129 strain of mice, and infrequently contribute to the germ line [4].
Another influence on the efficiency with which ES cells can be cultured from mouse blastocysts is the pregnancy status of the female. Pregnant mice that are in diapause tend to yield ES cells with greater success. Diapause occurs in female mice that have produced one litter and are still nursing when they become pregnant again. Diapause is a naturally occurring delay in the process of blastocyst implantation, which causes an arrest in embryonic development and a small increase in the number of epiblast cells [28]. These findings have led to the notion that genetic factors that are peculiar to specific strains of inbred mice, and other in vivo influences such as diapause, determine, to a great extent, whether mouse ES cells can be derived from a given blastocyst.
Generating cultures of mouse or human ES cells that remain in a proliferating, undifferentiated state is a multistep process that typically includes the following. First, the inner cell mass of a preimplantation blastocyst is removed from the trophectoderm that surrounds it. (For cultures of human ES cells, blastocysts are generated by in vitro fertilization and donated for research.) The small plastic culture dishes used to grow the cells contain growth medium supplemented with fetal calf serum, and are sometimes coated with a "feeder" layer of nondividing cells. The feeder cells are often mouse embryonic fibroblast (MEF) cells that have been chemically inactivated so they will not divide. Mouse ES cells can be grown in vitro without feeder layers if the cytokine leukemia inhibitory factor (LIF) is added to the culture medium (see below). Human ES cells, however do not respond to LIF.
Second, after several days to a week, proliferating colonies of cells are removed and dispersed into new culture dishes, each of which also contains an MEF feeder layer. Under these in vitro conditions, the ES cells aggregate to form colonies. Some colonies consist of dividing, nondifferentiated cells; in other colonies, cells may be differentiating. It is difficult to maintain human ES cells in dispersed cultures where cells do not aggregate, although mouse ES cells can be cultured this way. Depending on the culture conditions, it may also be difficult to prevent the spontaneous differentiation of mouse or human ES cells.
In the third major step required to generate ES cell lines, the individual, nondifferentiating colonies are dissociated and replated into new dishes, a step called passage. This replating process establishes a "line" of ES cells. The line of cells is "clonal" if a single ES cell generates it. Following some version of this fundamental procedure, human and mouse ES cells can be grown and passaged for two or more years, through hundreds of population doublings, and still maintain a normal complement of chromosomes, called a karyotype [31, 35].
Maintaining Mouse Embryonic Stem Cells in Their Undifferentiated State
Leukemia Inhibitory Factor and STAT3 Activation
Mouse ES cells can be maintained in a proliferative, undifferentiated state in vitro by growing them on feeder layers of MEF cells. An alternative to culture on feeder layers is the addition of leukemia inhibitory factor (LIF) to the growth medium [31, 39]. LIF is produced by feeder cells and, in their absence, allows mouse ES cells in vitro to continue proliferating without differentiating [20]. LIF exerts its effects by binding to a two-part receptor complex that consists of the LIF receptor and the gp130 receptor. The binding of LIF triggers the activation of the latent transcription factor STAT3, a necessary event in vitro for the continued proliferation of mouse ES cells [5, 12, 14]. Recent evidence indicates that two transcription factors, STAT3 and Oct-4, may interact and perhaps affect the function of a common set of target genes [15].
In vivo, signaling through the gp130 receptor is not necessary for normal, early embryonic development but is required to maintain the epiblast during diapause. After gastrulation, LIF signaling and STAT3 activation promote the differentiation of specific cell lineages such as the myeloid cells of the hematopoietic system or the astrocyte precursor cells in the central nervous system [9].
The self-renewal of mouse ES cells also appears to be influenced by SHP-2 and ERK activity. SHP-2 is a tyrosine phosphatase, an enzyme that removes phosphate groups to the tyrosine residues of various proteins. SHP-2 interacts with the intracellular (amino terminus) domain of the gp130 receptor. ERK (which stands for extracellular regulated kinase) is one of several kinds of enzymes that becomes activated when the gp130 receptor and other cell-surface receptors are stimulated. Both ERK and SHP-2 are components of a signal-transduction pathway that counteracts the proliferative effects of STAT3 activation. Therefore, if ERK and SHP-2 are active, they inhibit ES cell self-renewal [5] (see Figure B.2. The LIF-STAT3 Signaling Pathway Promotes Embryonic Stem Cell Self-Renewal).
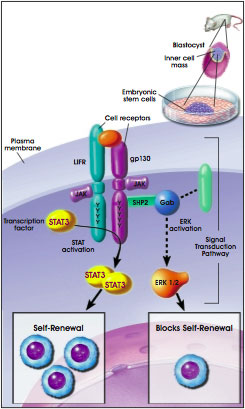
Figure B.2. The LIF-STAT3 Signaling Pathway Promotes Embryonic Stem Cell Self-Renewal.
(© 2001 Terese Winslow, Lydia Kibiuk)
It is possible that some of the components of signaling pathways in cultured mouse ES cells are unique to these cells. For example, mouse ES cells in vitro express high amounts of a modified version of an adapter protein, Gab1. The unusual form of Gab1 that occurs in ES cells may suppress interactions of specific receptors to the Ras-ERK signaling pathway [31]. Further, the expression of this altered form of Gab1 may be promoted by the transcription factor Oct-4. In mouse ES cells, Oct-4 expression and increased synthesis of Gab1 may help suppress induction of differentiation [30].
Thus, the emerging picture is that the effects of various signaling pathways must be balanced in a particular way for ES cells to remain in a self-renewing state. If the balance shifts, ES cells begin to differentiate [29, 30].
Expression of Oct-4 in Undifferentiated, Pluripotent Cells
One of the hallmarks of an undifferentiated, pluripotent cell is the expression of the Pou5f1 gene, which encodes the transcription factor Oct-4 (also called Oct-3 or Oct-3/4). Oct-4 is present in the mouse zygote, and is required throughout blastocyst development to establish [13] and maintain [15] the pluripotency of the inner cell mass and the epiblast. Oct-4 is also expressed in the primordial germ cells of mice and in mature germ cells [19, 23, 26].
Mouse ES cells in vitro can replicate indefinitely and produce 109 to 1010 (1 to 10 billion) cells without differentiating. in vitro, undifferentiated, proliferating mouse [18] and human [21] ES cells express Oct-4. Studies of Oct-4 expression and function in human cells are incomplete, however, and most of the information about Oct-4 comes from the study of mouse ES cells in vitro.
As is the case with inner cell mass and epiblast cells in vivo, Oct-4 expression in vitro is required to maintain the pluripotent, undifferentiated state of ES cells. If Oct-4 expression is inhibited in cultured mouse ES cells, the cells generate trophectoderm. If Oct-4 expression is artificially increased, mouse ES cells differentiate into primitive endoderm and mesoderm. Therefore, the level of Oct-4 expression dictates a significant aspect of the developmental program of mouse ES cells, making the protein a candidate "master regulator" of ES cell pluripotency [15].
How and why the Oct-4 transcription factor plays such an important role in early embryogenesis depend on the genes it regulates. Seven to eight target genes for Oct-4 have been identified to date; it activates some and represses others. In fact, the overall impact of Oct-4 may be to prevent the expression of genes that are required for differentiation [19].
The Cell Cycle of Mouse Embryonic Stem Cells: Its Role in Preventing Differentiation
Like the cells of the epiblast in the preimplantation mouse embryo, mouse ES cells in vitro have an unusual cell cycle. Specifically, the G1 checkpoint does not appear to operate in proliferating epiblast and ES cells [25, 38]. This may explain why it has not been possible to induce quiescence—withdrawal from the cell cycle to a G1 or G0 state—in undifferentiated ES cells [29].
However, if ES cells begin to differentiate by forming embryoid bodies, cyclin D expression increases, the G1 phase of the cell cycle becomes longer, and the rate of cell division slows [25]. This can occur if LIF or feeder layers are withdrawn from mouse ES cell cultures. Then, cell division continues for only a few days as the process of differentiation begins [29]. Perhaps constant cell proliferation somehow inhibits cell differentiation, and once the signals for cell division are removed, differentiation can occur [37].
Markers of Undifferentiated Embryonic Stem Cells
ES and EC cells, as well as cells of the inner cell mass of mouse blastocysts, express a panel of surface markers that are used to characterize undifferentiated, pluripotent embryonic cells. (see Table B.1. Comparison of Mouse, Monkey, and Human Pluripotent Stem Cells). The markers also distinguish mouse ES and EC cells from human ES and EC cells. For example mouse ES and EC cells express the stage-specific embryonic antigen SSEA-1, whereas human ES and EC cells do not. But human ES and EC cells express SSEA-3 and SSEA-4, whereas mouse ES and EC cells do not [21, 35].
Human EG cells, which are derived from primordial germ cells, express all three markers: SSEA-1, SSEA-3, and SSEA-4. The biological significance of the expression patterns of these surface antigens is unclear, but SSEA-1 expression may be related to the growth characteristic of the cells in vitro. Undifferentiated human ES and EC cells tend to grow in flat, relatively loose colonies. In contrast, mouse ES and EC colonies tend to be multilayered and compact [27]. Alternatively, the surface expression of various SSEAs may reflect a difference in the developmental stages of the cells [17].
Other markers used to identify ES cells are the surface antigens TRA1–60, TRA1–81, and the enzyme alkaline phosphatase. All occur in human ES [3, 27, 35], as they do in their mouse counterparts.
Genomic Imprinting in Embryonic Stem Cells
It is known that if genomic imprinting patterns are disturbed before blastocyst implantation in vivo, fetal abnormalities may result. In genomic imprinting, DNA methylation marks certain genes, depending on whether they are inherited from the mother or the father. The marked genes are turned on or off in a non-random pattern that is determined by parental origin. Imprinting marks are erased in the primordial germ cells and then reestablished during the formation of eggs and sperm.
However, when embryonic development occurs in vitro or when ES cells are grown in tissue culture, normal patterns of genomic imprinting may be disturbed. For example, mouse embryos that were grown in vitro in the presence of fetal calf serum—a very different environment than the oviduct—and then allowed to develop in vivo, showed abnormal genomic imprinting patterns and abnormal development. Apparently, the presence of fetal calf serum, a common ingredient in mouse and human ES cultures, decreases the expression of certain imprinted genes [8].
How or whether the use of fetal calf serum for culturing mouse or human ES cells affects genomic imprinting and the behavior of ES cells in vitro is not known. But for mouse ES cells, the parental imprinting pattern apparently persists in vitro [16, 22]. The imprinting pattern of human ES cells in vitro has not yet been determined.
Table B.1. Comparison of Mouse, Monkey, and Human Pluripotent Stem Cells
| Marker Name |
Mouse EC/
ES/EG cells |
Monkey
ES cells |
Human
ES cells |
Human
EG cells |
Human
EC cells |
| SSEA-1 |
+ |
– |
– |
+ |
– |
| SSEA-3 |
– |
+ |
+ |
+ |
+ |
| SEA-4 |
– |
+ |
+ |
+ |
+ |
| TRA-1–60 |
– |
+ |
+ |
+ |
+ |
| TRA-1–81 |
– |
+ |
+ |
+ |
+ |
| Alkaline phosphatase |
+ |
+ |
+ |
+ |
+ |
| Oct-4 |
+ |
+ |
+ |
Unknown |
+ |
| Telomerase activity |
+ ES, EC |
Unknown |
+ |
Unknown |
+ |
| Feeder-cell dependent |
ES, EG, some EC |
Yes |
Yes |
Yes |
Some; relatively low clonal efficiency |
| Factors which aid in stem cell self-renewal |
LIF and other factors that act through gp130 receptor and can substitute for feeder layer |
Co-culture with feeder cells; other promoting factors have not been identified |
Feeder cells + serum; feeder layer + serum-free medium + bFGF |
LIF, bFGF, forskolin |
Unknown; low proliferative capacity |
| Growth characteristics in vitro |
Form tight, rounded, multi-layer clumps; can form EBs |
Form flat, loose aggregates; can form EBs |
Form flat, loose aggregates; can form EBs |
Form rounded, multi-layer clumps; can form EBs |
Form flat, loose aggregates; can form EBs |
| Teratoma formation in vivo |
+ |
+ |
+ |
– |
+ |
| Chimera formation |
+ |
Unknown |
+ |
– |
+ |
KEY
ES cell = Embryonic stem cell
EG cell = Embryonic germ cell
EC cell = Embryonal carcinoma cell
SSEA = Stage-specific embryonic antigen |
TRA = Tumor rejection antigen-1
LIF = Leukemia inhibitory factor
bFGF = Basic fibroblast growth factor
EB = Embryoid bodies |
Targeted Differentiation of Mouse Embryonic Stem Cells.
Outlined here are three different ways to direct mouse ES cell differentiation in vitro. In the first example, mouse ES cells are directed to generate primitive blood vessels. In the second, mouse ES cells are directed to become neurons that release the transmitters dopamine and serotonin. And in the third—a series of experiments conducted by the same lab group that generated dopamine neurons—very similar conditions are used to direct the differentiation of mouse ES cells to yield pancreatic islet cells that secrete insulin.
Making Vascular Progenitors from Mouse Embryonic Stem Cells
In the mouse embryo, blood cells and blood vessels are formed at roughly the same time, when blood islands first appear in the wall of the yolk sac. A prevailing idea is that blood cells and blood vessels arise from a common precursor cell derived from mesoderm, the hemangioblast. After hemangioblasts differentiate from the mesoderm, they aggregate to form blood islands. The inner cells of the blood islands become hematopoietic stem cells, or blood-forming cells. The outer cells of the blood islands become angioblasts, which give rise to the blood vessels. A recent study showed that mouse ES cells in vitro could be induced to follow this in vivo developmental pathway.
In vivo, blood vessel formation occurs in two ways: by vasculogenesis and angiogenesis. Vasculogenesis helps establish the blood islands and the capillary network that connects them. During angiogenesis, new blood vessels form by remodeling or adding to existing vessels. Both vasculogenesis and angiogenesis are regulated by the actions of a series of paracrine growth factors, which include fibroblast growth factor–2 (FGF-2), vascular endothelial growth factor (VEGF), and later (in the adult) platelet-derived growth factor (PDGF) and transforming growth factor beta (TGFß). Each of these growth factors binds to specific receptors. VEGF, for instance, binds to two different receptors: VEGF-R1, also known as Flt1, and VEGF-R2, also known as Flk1 [7].
To make vascular progenitors from mouse ES cells, Shin-Ichi Nishikawa of Kyoto University Graduate School of Medicine in Japan and his colleagues tried to mimic this in vivo pathway for blood vessel formation [40]. They grew undifferentiated ES cells on collagen-coated dishes in medium containing fetal calf serum but no leukemia inhibitory factor (LIF). This induced the generation of cells that express Flk1, a receptor for VEGF. Several days later, the cells began to differentiate. Nearly all the mouse ES cells expressed α-smooth muscle actin (SMA), a marker for mural cells. (Mural cells, which include pericytes and smooth muscle cells, normally interact in vivo with endothelial cells to make blood vessels.) When VEGF was added to the culture medium, sheets of endothelial cells formed that expressed platelet-endothelial cell adhesion molecule (PECAM1) and other endothelial cell markers. At this point, the culture contained two differentiating cell types, endothelial cells and mural cells.
Therefore, it appeared that the mouse ES cells had differentiated into Flk1+ precursor cells, which then gave rise to both mural cells and endothelial cells in vitro. To test that hypothesis, single Flk1+ cells were cultured. The individual ES cells generated three kinds of colonies: pure mural cells (SMA+), pure endothelial cells (PECAM1+), and mixed mural and endothelial cells. That result indicated that ES cells can give rise to Flk1+ cells that are precursors for both mural and endothelial cells.
The next test was to see whether the mural cells and endothelial cells generated from Flk1+ precursors could assemble into primitive blood vessels in vitro. They did. By growing hundreds of Flk1+ cells in collagen gel suspensions with fetal calf serum and VEGF, tube-like structures formed within three to five days. This change in the culture conditions allowed the ES cells to grow in suspension and interact with each other. As a result, the cells spontaneously organized themselves into tube-like structures that resemble blood vessels in vivo. The tubes were composed of endothelial (PECAM1+) cells and mural (SMA+) cells. Occasionally, they formed branching structures, which is typical of the organization of blood vessels in vivo. Also, blood cells (bearing the markers CD45 and Ter119) formed inside the tubes, which also mimicked the organization of blood islands in the early embryo in vivo.
The final test was to see whether the Flk1+ cells generated from mouse ES cells in vitro would differentiate into endothelial cells and mural cells in vivo. Again, they did. Flk1+ cells were engineered to express LacZ (which allows the cells to be tracked visually) and injected into the developing hearts of stage 16–17 chick embryos. The donor mouse cells populated blood vessels in the chicks' head, yolk sac, heart, and regions between the somites, forming endothelial cells and mural cells in those regions.
Thus, undifferentiated mouse ES cells can be directed to differentiate into Flk1+ precursors that give rise to endothelial cells and mural cells in vitro and in vivo. Further, the differentiated cells can form tube-like vascular structures in vitro. The experiments not only demonstrate the power of directed differentiation of ES cells into individual cell types, they also show that ES cells can generate multiple cell types that then spontaneously organize themselves into tissues that resemble those in vivo. In addition, the experiments by Nishikawa and his co-workers [40] reveal that Flk1+ cells are important for generating blood vessels in vivo.
Making Dopamine Neurons from Mouse Embryonic Stem Cells
A second example of the directed differentiation of mouse ES cells in vitro yielded the formation of particular kinds of neurons that normally occur in the mammalian midbrain and hindbrain. For a long time, the goal of efficiently inducing the formation of these neurons—which release the neurotransmitters dopamine and serotonin, respectively—was highly desired, but elusive. In Parkinson's Disease, a key population of midbrain neurons that releases dopamine dies. So finding a way to grow large quantities of nerve cells in vitro that might be able to replace lost dopamine neurons in vivo is a clinical priority (see Chapter 8. Rebuilding the Nervous System with Stem Cells).
Last year, Ron McKay and his colleagues reported an efficient technique for inducing mouse ES cells in vitro to differentiate into dopamine neurons of the midbrain and serotonin neurons, which normally populate the hindbrain. Like Nishikawa and his colleagues [40], McKay and his collaborators [10] triggered the differentiation of mouse ES cells in vitro at various stages by changing the growth conditions to mimic, in part, those that occur during embryogenesis in vivo. The resulting differentiated nerve cells look and function like their in vivo counterparts.
During embryogenesis, central nervous system (CNS) development is a long, complex process that depends on a highly coordinated series of cellular and molecular events. Different signals direct the formation of the neurectoderm from the epiblast, a process that ultimately results in the formation of the CNS, the brain and spinal cord. Later, other signals regulate the development of different parts of the brain. For example, early in the formation of the brain, the homeobox genes OTX1 and OTX2 are expressed [28]. Cells of the epiblast express OTX2 before the onset of gastrulation. Then, during gastrulation, OTX2 is expressed in the anterior neurectoderm, where it is necessary for the formation of the midbrain and forebrain. Meanwhile, OTX1 expression occurs in the region of the neurectoderm that gives rise to the dorsal forebrain. Interactions between OTX1 and OTX2 are thought to help shape the midbrain and hindbrain [1].
Once these major brain structures form, various genes control the development of individual nerve cell types. For example, the genes Pax2, Pax5, Wnt1, En1, and Nurr1 help control the differentiation of neurons that release the transmitters dopamine and serotonin [24, 33]. Furthermore, when the proteins sonic hedgehog (SHH) and fibroblast growth factor-8 (FGF-8) are added to explant cultures (small chunks of tissue maintained in vitro) of neural plate, the development of midbrain neurons is enhanced [41].
Taking into account these and other findings, McKay and his coworkers devised an in vitro system for controlling the differentiation of mouse ES cells into midbrain neurons that release dopamine and hindbrain neurons that release serotonin [11]. The culture conditions they used differ from those devised by Nishikawa and his colleagues (described above), but the starting material—undifferentiated, proliferating mouse ES cells—was the same in both experiments. McKay and his colleagues cultured mouse ES cells in five distinct stages, each of which they identified by the changes in culture conditions and the behavior of the cells (see Figure B.3. Directed Differentiation of Mouse Embryonic Stem Cells Into Neurons or Pancreatic Islet-Like Clusters).
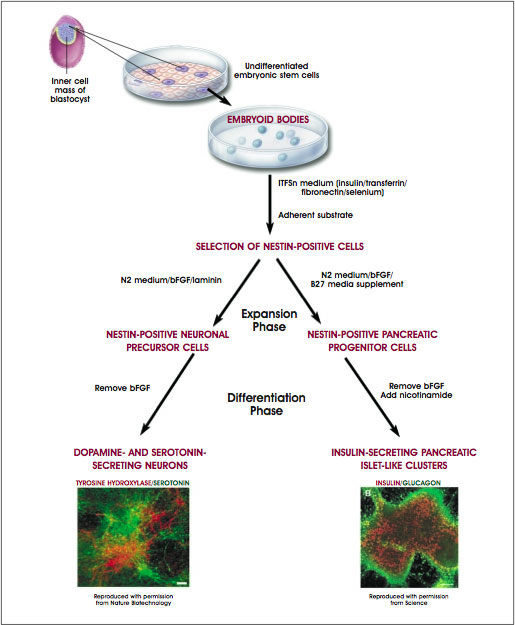
Figure B.3. Directed Differentiation of Mouse Embryonic Stem Cells Into Neurons or Pancreatic Islet-Like Clusters.
(© 2001 Terese Winslow, Caitlin Duckwall)
In stage 1, undifferentiated mouse ES cells were dissociated into single cells and plated at low density. They proliferated in plastic culture dishes coated with gelatin. The growth media contained LIF and fetal calf serum and was supplemented with amino acids, conditions that promoted the proliferation of undifferentiated ES cells. In stage 2, the cells were induced to form embryoid bodies by dissociating them and replating at a higher density on a nonadherent surface. These conditions allowed the cells to aggregate and begin the process of differentiation. After four days, the cells were replated on an adherent substrate in the original (stage 1) growth medium. Twenty-four hours later, the growth medium was replaced with serum-free insulin/transferrin/selenium/fibronectin (ITSFn) medium. This switch to a serum-free medium (one lacking fetal calf serum) caused many cells to die but allowed the survival of cells that express nestin. This intermediate filament protein is used as a marker to identify CNS stem cells in vivo and in vitro, although it is also expressed by other cell types. Stage 1 neurons expressed high levels of OXT2, which decreased in stages 2 and 3. OXT1 was not expressed until the cells reached stage 3.
Guiding the mouse ES cells through stages 4 and 5 of in vitro development yielded fully differentiated dopaminergic and serotoninergic neurons. After 6 to 10 days in the medium that selects for cells that express nestin, the cells were dissociated and induced to divide in another medium, N2, supplemented with laminin and basic FGF, a growth factor that induces proliferation. Other critical additives to yield stage 4 cells were SHH and FGF-8. Cells at stages 3 and 4 express genes that, in vivo, trigger the development of dopaminergic and serotinergic neurons—namely, Pax2, Pax5, Wnt1, En1, and Nurr1. Stage 5, the final stage of differentiation, was achieved by removing basic FGF from the growth medium (which made the cells stop dividing) and growing the cells for 6 to 15 days in N2 medium supplemented with laminin and ascorbic acid, a combination of additives that induces the differentiation of serotonin neurons.
This complex, multistage differentiation process yielded a higher percentage (30 percent) of neurons that express tyrosine hydroxylase (TH, the rate-limiting enzyme in the synthesis of dopamine) than any other reported in vivo or in vitro technique. The cells were confirmed to be true dopamine neurons by several functional assays. The neurons secreted dopamine into the culture medium, showed the electrical activity typical of neurons, and responded to the addition of a high concentration of potassium ions (via the addition of potassium chloride) by releasing more dopamine, much as they would in vivo. A separate population of neurons in the mouse ES cell cultures stained positive for serotonin. The differentiation of serotoninergic neurons could be induced by adding only SHH to the culture medium; addition of FGF-8 was not required. Thus, mouse ES cells in vitro can be directed to differentiate at a high efficiency into neurons that release either dopamine or serotonin.
Making Pancreatic Islet Cells from Mouse Embryonic Stem Cells
The experimental strategy is similar to that described above [10] and is based on a five-stage, in vitro system. As before (to differentiate neurons that produce dopamine), undifferentiated mouse ES cells are induced to proliferate in LIF-supplemented medium (stage 1). Then, the cells are induced to form embryoid bodies (EBs) in serum-free ITSFn medium without LIF (stage 2). ES stage 1 cells expressed Oct-4, a transcription factor that characterizes undifferentiated, proliferating, pluripotent cells. Again, cells that express nestin survive in serum-free medium, whereas other cell types do not, thus creating an environment that "selects" for nestin-positive cells (stage 3). As before, cells that express nestin are expanded by adding the mitogen basic FGF to the serum-free medium (stage 4). When basic FGF is withdrawn, the cells stop dividing and begin their final stages of differentiation. It is at this point that the techniques for generating neurons that release dopamine and pancreatic islet cells that release insulin diverge.
To generate neurons that release dopamine, ES-derived cells were cultured in medium that contained SHH and FGF-8 and later, an N2 medium supplemented with laminin and ascorbic acid [10]. To generate pancreatic islet cells, however, B27 culture medium was used for stage 4, and nicotinamide was added to stage 5 cultures. Another change in the pancreatic islet culture system was to co-culture individual stage 4 or 5 ES cells, which were tagged with the marker green fluorescent protein (GFP), with nontagged ES cells. This meant that an individual, tagged ES cell could be followed so its developmental fate could be traced, a technique that made possible the clonal analysis of the labeled ES cell and its progeny. Tagged, GFP-expressing ES cells gave rise to both pancreatic islet cells and neurons, indicating that the same cell acted as the precursor for both differentiated cell types (see Figure B.3. Directed Differentiation of Mouse Embryonic Stem Cells Into Neurons or Pancreatic Islet-Like Clusters).
The tests that identified the differentiated cells types as pancreatic islet cells and neurons included assays of various markers. The ES cells at stages 1 and 5 expressed GATA-4 and HNFb, markers for embryonic endoderm and extra-embryonic endoderm. This indicates that endodermal markers are present in undifferentiated ES cells. But stage 5 cells express additional markers that are characteristic of endocrine pancreatic islet cells: mouse insulin I and II, islet amyloid polypeptide, and the glucose transporter GLUT-2. Other cells stained positive for glucagon, a hormone produced by the alpha cells of the pancreas, and somatostatin, a peptide hormone produced by pancreatic endocrine cells in vivo. Nerve cells that surrounded the clusters of islet cells—a spontaneously forming, in vitro arrangement of cell types that mimicked their arrangement in vivo—stained positive for neuron-specific tubulin. No cells stained positive for both pancreatic islet markers and neuronal markers, indicating that the two cell types had differentiated completely from a common precursor cell.
Other tests demonstrated the functional properties of the pancreatic islet cells differentiated from mouse ES cells. Adding glucose to the culture medium triggered the release of insulin in a dose-dependent manner. Agonists and antagonists of insulin release in vivo stimulated or blocked insulin release in vitro, indicating that the pharmacological responses of the ES-derived islet cells in vitro mirrored in vivo responses. Finally, when cell clusters of the cultured pancreatic islets were grafted under the skin of diabetic mice (whose diabetes was induced by treatment with streptozocin), the grafts survived and became infiltrated with blood vessels. The ES-derived pancreatic islets released only one-fiftieth the amount of insulin they released as islet cells in vivo, however, the diabetic mice experienced no correction of their hyperglycemia (see Chapter 7. Stem Cells and Diabetes; and Figure 7.2. Development of Insulin Secreting Pancreatic-Like Cells from Mouse Embryonic Stem Cells).
Taken together, the three studies show that the differentiation of lines of mouse ES cells can be directed in vitro to yield vascular structures [40], neurons that release dopamine and serotonin [10], and endocrine pancreatic islet cells. In all three cases, proliferating, undifferentiated mouse ES cells provided the starting material and functional, differentiated cells were the result. Also, in all three experiments, the onset of mouse ES cell differentiation was triggered by withdrawing the cytokine LIF, which promotes the division of undifferentiated mouse ES cells, but—inexplicably—does not have the same effect on human ES cells. In addition, the ES cells in all three examples cited were induced to aggregate, a change in their three-dimensional environment that presumably allowed some of the cell-cell interactions to occur in vitro that would occur in vivo during normal embryonic development.
Collectively, these three studies provide some of the best examples of directed differentiation of mouse ES cells in vitro. Two of them showed that a single precursor cell can give rise to multiple, differentiated cell types [10, 40], and all of three studies demonstrated that the resulting differentiated cells function as their in vivo counterparts do.
These two criteria—demonstrating that a single cell can give rise to multiple cells types (clonal analysis), and that the functional properties of the differentiated cells—form the basis of an acid test for all claims of directed differentiation of either ES cells or of adult stem cells. Unfortunately, very few experiments meet these criteria, which too often makes it impossible to assess whether a differentiated cell type resulted from the experimental manipulation that was reported.
References
- Acampora, D. and Simeone, A. (1999). The TINS Lecture. Understanding the roles of Otx1 and Otx2 in the control of brain morphogenesis. Trends. Neurosci. 22, 116–122.
- Andrews, P.W., Casper, J., Damjanov, I., Duggan-Keen, M., Giwercman, A., Hata, J., von Keitz, A., Looijenga, L.H., Millan, J.L., Oosterhuis, J.W., Pera, M., Sawada, M., Schmoll, H.J., Skakkebaek, N.E., van Putten, W., and Stern, P. (1996). Comparative analysis of cell surface antigens expressed by cell lines derived from human germ cell tumours. Int. J. Cancer. 66, 806–816.
- Andrews, P.W., Damjanov, I., Simon, D., Banting, G.S., Carlin, C., Dracopoli, N.C., and Fogh, J. (1984). Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab. Invest. 50, 147–162.
- Brook, F.A. and Gardner, R.L. (1997). The origin and efficient derivation of embryonic stem cells in the mouse. Proc. Natl. Acad. Sci. U. S. A. 94, 5709–5712.
- Burdon, T., Chambers, I., Stracey, C., Niwa, H., and Smith, A. (1999). Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs. 165, 131–143.
- Evans, M.J. and Kaufman, M.H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature. 292, 154–156.
- Gilbert, S.F. (2000). Developmental biology. (Sunderland, MA: Sinauer Associates).
- Khosla, S., Dean, W., Brown, D., Reik, W., and Feil, R. (2001). Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol. Reprod. 64, 918–926.
- Kishimoto, T., Taga, T., and Akira, S. (1994). Cytokine signal transduction. Cell. 76, 253–262.
- Lee, S.H., Lumelsky, N., Studer, L., Auerbach, J.M., and McKay, R.D. (2000). Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 18, 675–679.
- Martin, G.R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U. S. A. 78, 7634–7638.
- Matsuda, T., Nakamura, T., Nakao, K., Arai, T., Katsuki, M., Heike, T., and Yokota, T. (1999). STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 18, 4261–4269.
- Nichols, J., Zevnik, B., Anastassiadis, K., Niwa, H., Klewe-Nebenius, D., Chambers, I., Scholer, H., and Smith, A. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 95, 379–391.
- Niwa, H., Burdon, T., Chambers, I., and Smith, A. (1998). Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12, 2048–2060.
- Niwa, H., Miyazaki, J., and Smith, A.G. (2000). Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 24, 372–376.
- O'Shea, K.S. (1999). Embryonic stem cell models of development. Anat. Rec. 257, 32–41.
- Pera, M., personal communication.
- Pesce, M., Gross, M.K., and Scholer, H.R. (1998). In line with our ancestors: Oct-4 and the mammalian germ. Bioessays. 20, 722–732.
- Pesce, M., Wang, X., Wolgemuth, D.J., and Scholer, H. (1998). Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech. Dev. 71, 89–98.
- Rathjen, P.D., Toth, S., Willis, A., Heath, J.K., and Smith, A.G. (1990). Differentiation inhibiting activity is produced in matrix-associated and diffusible forms that are generated by alternate promoter usage. Cell. 62, 1105–1114.
- Reubinoff, B.E., Pera, M.F., Fong, C.Y., Trounson, A., and Bongso, A. (2000). Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 18, 399–404.
- Rohwedel, j., Sehlmeyer, U., Shan, J., Meister, A., and Wobus, A.M. (1996). Primordial germ cell-derived mouse embryonic germ (EG) cells in vitro resemble undifferentiated stem cells with respect to differentiation capacity and cell cycle distribution. Cell. Biol. Int. 20, 279–587.
- Rosner, J.L. (1990). Reflections of science as a product. Nature. 345, 108.
- Rowitch, D.H. and McMahon, A.P. (1995). Pax-2 expression in the murine neural plate precedes and encompasses the expression domains of Wnt-1 and En-1. Mech. Dev. 52, 3–8.
- Savatier, P., Lapillonne, H., van Grunsven, L.A., Rudkin, B.B., and Samarut, J. (1996). Withdrawal of differentiation inhibitory activity/leukemia inhibitory factor up-regulates D-type cyclins and cyclin-dependent kinase inhibitors in mouse embryonic stem cells. Oncogene. 12, 309–322.
- Schöler, H.R., Ruppert, S., Suzuki, N., Chowdhury, K., and Gruss, P. (1990). New type of POU domain in germ line-specific protein Oct-4. Nature. 344, 435–439.
- Shamblott, M.J., Axelman, J., Wang, S., Bugg, E.M., Littlefield, J.W., Donovan, P.J., Blumenthal, P.D., Huggins, G.R., and Gearhart, J.D. (1998). Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. U. S. A. 95, 13726–13731.
- Simeone, A. (1998). Otx1 and Otx2 in the development and evolution of the mammalian brain. EMBO J. 17, 6790–6798.
- Smith, A.G. (2001). Embryonic stem cells. Marshak, D.R., Gardner, D.K., and Gottlieb, D. eds. (Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press). 205–230.
- Smith, A., personal communication.
- Smith, A.G. (2001). Origins and properties of mouse embryonic stem cells. Annu. Rev. Cell. Dev. Biol.
- Stevens, L.C. (1970). The development of transplantable teratocarcinomas from intratesticular grafts of pre-and postimplantation mouse embryos. Dev. Biol. 21, 364–382.
- Stoykova, A. and Gruss, P. (1994). Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J. Neurosci. 14, 1395–1412.
- Thomson, J.A., Kalishman, J., Golos, T.G., Durning, M., Harris, C.P., Becker, R.A., and Hearn, J.P. (1995). Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. U. S. A. 92, 7844–7848.
- Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S., and Jones, J.M. (1998). Embryonic stem cell lines derived from human blastocysts. Science. 282, 1145–1147.
- Thomson, J.A. and Marshall, V.S. (1998). Primate embryonic stem cells. Curr. Top. Dev. Biol. 38, 133–165.
- Weissman, I.L. (2000). Stem cells: units of development, units of regeneration, and units in evolution. Cell. 100, 157–168.
- Wianny, F., Real, F.X., Mummery, C.L., van Rooijen, M., Lahti, J., Samarut, J., and Savatier, P. (1998). G1-phase regulators, Cyclin D1, Cyclin D2, and Cyclin D3: up-regulation at gastrulation and dynamic expression during neurolation. Dev. Dyn. 212, 49–62.
- Williams, R.L., Hilton, D.J., Pease, S., Willson, T.A., Stewart, C.L., Gearing, D.P., Wagner, E.F., Metcalf, D., Nicola, N.A., and Gough, N.M. (1988). Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 336, 684–687.
- Yamashita, J., Itoh, H., Hirashima, M., Ogawa, M., Nishikawa, S., Yurugi, T., Naito, M., Nakao, K., and Nishikawa, S. (2000). Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 408, 92–96.
- Ye, W., Shimamura, K., Rubenstein, J.L., Hynes, M.A., and Rosenthal, A. (1998). FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 93, 755–766.
 Appendix A | Table of Contents | Appendix C
Appendix A | Table of Contents | Appendix C 
Historical content: June 17, 2001