The NIH will provide state-of-the-art animal research facilities to enhance and maintain its position as the world leader in biomedical research. The NIH will accomplish this goal by constructing new animal research facilitiesand renovating older ones to meet ever-changing biomedical research requirements. These guidelines will be applied to new animal research facilities and, to the extent possible, to renovation projects. The following goals and objectives define the minimum recommended program requirements and recommendations for the design of animal research facilities. For specific requirements, refer to Animal Research Facilities, Section: Design Criteria.On this page:
B.1 Program Objectives
B.2 Quality of Life and Environmental Considerations
B.3 Animal Research Facility Space
B.4 Flexibility and Adaptability
B.5 Planning Module
B.6 Functional Relationships and Zoning of the Animal Research Facility
B.7 Circulation of People, Animals, and Materials
B.8 Security
B.9 Loading Docks
B.1 Program Objectives
Program objectives must be determined as early as possible in the planning process. It is crucial to identify the variety of species that the facility should be expected to accommodate over time, the temperature and humidity range that each species can tolerate, and the degree of flexibility and adaptability required within the facility to accommodate different species. The designer should determine the cost impact of making some or all areas of the facility more flexible than others. In order to provide for an environment within the animal research facility that meets the program objectives, the designer will collect data on spatial allocations, functional adjacen-cies, user requirements, staffing projections, flexibility requirements, redundancy requirements, security requirements, architectural finishes, fixed equipment needs and circulation of personnel, material, animals, and waste.
Early in the planning process, the designer should work with the facility representatives to prepare a functional and adjacencies flow chart that will facilitate the design process. In addition to impacting the ease of doing animal-model-based science, the arrangement of critical adjacencies will greatly impact the quality of life of the animals, the caretakers, and the veterinarians. Appropriate adjacency planning will modify interference from noise and vibrations, economize circulation routes, and maintain the appropriate degree of cleanliness of the facility.
B.1.1 Planning Criteria: The animal research facility will be designed to house animals in an appropriate species-specific environment that meets or exceeds all applicable policies, guidelines, and regulations as outlined in the Guide for the Care and Use of Laboratory Animals (Guide), Public Health Service (PHS) policy, and animal welfare regulations. In addition, facilities must meet the minimum requirements to be accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The ideal facility will:
- Meet projected holding and programmatic requirements while providing for expansion and flexibility in space utilization
- Provide for efficiency of management through innovation and flexible design
- Be cost-effective in design, construction, operation, and maintenance
- Utilize innovative design and construction to minimize future energy, maintenance, labor, and expansion costs
- Provide an ergonomic and user-friendly work environment
top
B.2 Quality of Life and Environmental Considerations
It is important to recognize that the immediate environment directly and indirectly affects an animal’s biological and behavioral responses. Not only does the environment in which they live (i.e., noise, light, vibration, sound, ventilation, etc.) affect the quality of life, but these factors also may adversely impact the research being conducted. At a minimum, animal holding and procedure areas must be designed to ensure animal well-being, meet research requirements, be able to be sanitized and easily maintained, and minimize experimental variables or maximize predictability.
B.2.1 Animal Well-Being: The facility should support a healthy social environment for the animals that mimics the animal’s natural social environment. The characteristics of each species must be considered in deciding how to house a diverse animal species. There are few data comparing the relationship of quality or quantity of an animal’s activity to its physical or psychological well-being. A cage does not necessarily limit the amount of animal activity, although it may alter the form of activity the animal can pursue. The need for exercise or induced activity is subject to the judgment of the animal science professional based on an understanding of the species or breed; its temperament, age, history, and physical condition; nature of the research; and expected duration of animal research facility residence. Examples of supplementary activity include running on a treadmill or exercise wheel, walking on a leash, having access to a run, or moving an animal from its cage into an animal playroom/activity area. Provision shall be made for animals with specialized locomotion patterns to express these patterns, especially when animals are held for long periods. For example, ropes, bars, and perches are appropriate for brachiating nonhuman primates.
B.2.2 Employee Well-Being: The animal research facility is also a workplace for human employees. Therefore, the occupational health and safety of personnel must be considered. The environment shall be aesthetically pleasing to employees and consistent with the needs of investigators engaged in animal research. It shall be efficient, secure, and easy to maintain and perform animal caretaking services. Sufficient air supply, filtration, and exhaust shall be provided to minimize unpleasant animal odors and animal allergens. Provision of natural light (if structurally feasible), adequate work space, color, and ergonomic furniture systems are integral to a pleasing, functional, and effective work environment that will enhance productivity and aid in the recruitment and retention of quality personnel. In order to provide for an environment within the animal research facility that meets these goals, see Animal Research Facilities, Section: Space Descriptions, for information that impacts the quality of life of the animals and their caretakers.
B.2.2.1 Graphics/Signage: Without views to the outside or significant landmarks within the facility, orientation becomes a planning issue in an animal facility. It is recommended that a map of the corridor system be provided at strategic junctures in the hallways. Alternative wayfinding elements might be used such as directional markers on the walls or color-coded corridors or artistic symbols designating room or corridor use. Each room shall have a room number clearly displayed at its entry.
B.2.2.2 Other Amenities: Amenities such as lounges, break areas, training rooms, staff offices, and conference rooms shall be provided. Placement and size of these rooms shall be carefully thought out in order to maintain the integrity of the degree of facility contamination control that is defined in the program. Locker and shower facilities should be provided outside the animal barrier area for staff whose work does not involve animal contact.
B.2.3 Natural Light: Natural light is not recommended in areas that will house animals that require regulated lighting cycles. These include but are not limited to rodents, rabbits, and fish. Windows may be desired in areas that house large animals such as nonhuman primates, dogs, or farm animals. With the exception of facilities housing larger farm species, most animal rooms should be equipped with artificial lighting systems that control the diurnal lighting cycle. Through the use of innovative design and construction, diurnal variation can be maintained. If windows are to be placed in animal rooms, veterinarians shall be consulted for placement of windows and window treatments. The negative aspects of windows in animal facilities frequently dominate design, and opportunities can be missed to enhance the work environment with natural lighting. Where possible, windows should be provided in personnel and administrative areas.
B.2.4 Lighting: Fluorescent lighting is recommended in an animal facility. However, discussions shall be held with the veterinarian and researchers regarding the light spectrum and light covers of fluorescent lamps. Light covers should diffuse and soften the light so as to have a minimal effect on animals that may have higher than normal light sensitivity. Lighting should be waterproof, recessed, ceiling mounted, and sealed and caulked top prevent vermin infestation.
Lighting control is a major consideration, particularly in small-animal holding rooms. Lighting control may be required for large-animal holding or procedure rooms as well. Light intensity can have an impact on research results under certain circumstances and may differ by species. Whenever possible, lighting should be centrally controlled and monitored at the room level. Monitoring of the lighting control system should be independent from the method used to control the lights. Consideration should be given to direct measurement of room illumination or monitoring the electrical circuit feeding the room light. The ideal system would provide a local warning light alarm and, if required, remote audible alarms signaling lighting failures. Although it may be possible to group several rooms on a single timer, this should be discussed with the users. Animal protocols often call for diurnal lighting cycles (circadian rhythm) to be reversed or altered in duration for the researchers’ needs or for the desired results of the experiment. These studies require lighting controls and automatic timers in all holding rooms and isolation cubicles. “Red light” or other lighting options within holding rooms, as determined by users, should be considered so researchers can enter a room during the dark cycle without affecting the animals.
Consideration should be given to providing a warning light outside the rooms that are on automatic timed lighting to indicate that the room is on the dark cycle.
B.2.5 Noise: Acoustical control is an important planning consideration and shall be evaluated during design. By examining adjacencies, the effects of noise can be addressed in the design layout. Most animals are stimulated and may be stressed by noise. Different species of animals will have different tolerances for high- or low-frequency noises. Certain frequencies can have an adverse affect on sensitive animals. These issues must be discussed with facility users.
Ventilated racks generate noise. The rack density in a room will affect the noise level. Mechanical equipment may generate noise frequencies that are not noticeable to humans but will potentially affect animals housed near the source of the noise. Equipment that generates noise should be remote or acoustically isolated from animal holding rooms wherever possible.
Large animals tend to be noisier than small animals, although avian species (birds) are noisier in relation to their size than rodents. Animal species that generate noise should be isolated from those that are noise sensitive by either distance or sufficient acoustical isolation. Noise conductivity through the duct system should be taken into consideration.
Although rodents can adjust to constant, low-level background noise, it should be minimized or removed through the use of innovative design.
In all situations, it is imperative to eliminate the effects of sudden and variable noiseproducing elements, such as fire alarms, throughout the animal holding environments. Strobe lights must be used in mouse breeding facilities.
B.2.6 Vibration Stability: Vibration stability is important to maintaining a constant experimental environment for sensitive animals such as rodents. Therefore, rodent holding and test rooms should be located away from areas such as a cagewash, major circulation corridors where racks are frequently in transit, mechanical rooms, and elevator shafts. Vibration is not as much of an issue for large animals except in behavior testing rooms. Vibration studies should be performed to determine how best to achieve the maximum allowable vibration levels as determined by instruments and animals to be used in the area.
Vibration stability should be considered in an animal facility where specialized equipment will be used such as animal imaging equipment, electron microscopy, and electrophysiology procedures, including intracellular data collection equipment.
Vibration stability will be of greater concern if the animal facility is located on the upper levels of a building rather than at ground level because of structural considerations. Sensitive pieces of equipment may require an isolation slab. Some equipment can be stabilized using a dampening device.
top
B.3 Animal Research Facility Space
B.3.1 Ratio of Holding Rooms to Procedure Rooms: During the programming stage, the users should be consulted on whether animal holding rooms will house multiple species and whether animal holding rooms and procedure rooms should be designed to be interchangeable with minimal structural modification. Flexibility in design of these critical areas provides for rapid accommodation of future programmatic changes and efficient space utilization.
As a general rule of thumb, one procedure room for every three to four small-animal holding rooms should be considered. Clusters of isolation cubicles should have at least one procedure room per cluster. Suites should have a minimum of one procedure room within the suite. The ratio of procedure rooms to holding rooms for large animals shall be determined by scientific requirements and the specific program requirements of the facility.
Most large-animal holding facilities will require an extensive surgical suite with its accompanying specialty procedure and prep rooms. Terminal procedures and autopsies on large animals are ideally conducted in separate locations from the surgical suite but can be performed in a necropsy/perfusion room or a specially designated procedure room.
B.3.2 Animal Research Facility Support Space: The ratio of animal facility support space to holding and procedure space is generally 2:1 or higher. Support space includes bedding and feed storage, decontamination areas for incoming animals and materials, a laundry, feed preparation areas, administrative areas, break areas, meeting/training rooms, lockers for animal caretakers, gowning areas, cagewash, autoclaves, marshalling areas, diagnostic laboratories, pharmacy, storage areas, and housekeeping closets. Storage areas should include space for cage and rack equipment, general support supply storage, and locked cabinets where researchers can store small research-specific items.
Shared-use spaces may include surgical suites, imaging suites, behavioral suites, transgenic suites, radiology rooms, and irradiator(s), in addition to the operational support spaces. Shared-use, central, or core facilities may be considered as part of support space or as part of procedure/holding space depending on how the program chooses to define the space. The definition of this space should be established early in the programming process to facilitate net-to-gross calculations.
B.3.3 Office and Administrative Space: Animal facility administrative areas should be designed using standard administrative space parameters. There may be a programmatic need for separate offices for Government and contract supervisory staff. Administration offices should be located near the main animal facility entrance. This locates the management personnel in a position to observe the movement of personnel and equipment into or out of the facility. Guests, vendors, and service people should have access to the animal facility administrative areas without entering the animal housing and support areas. Ideally, each veterinarian, manager, and, if required, Contract Project Officer should have a private office. Space should be allowed for office equipment such as copying machines and fax machines. A separate area for housing centralized computers and monitoring equipment should be considered. The administrative area shall include conference rooms, a break room, and access to toilet facilities that are separate from those used by the animal caretakers.
B.3.4 Area Allowances: The following calculations are for the purposes of design and construction and not to determine real estate space allocations for purposes of charging rent. See Volume: Appendices for information on the methodology used to calculate gross and net area.
B.3.4.1 Grossing Factor: For budget purposes, the gross building area generally includes the total area of all spaces, including basements, mezzanines, penthouses, mechanical, electrical, and communications spaces, and enclosed loading docks. The gross building area will exceed the net area by a grossing factor. A range is given for these factors,depending on design choices for internal circulation patterns, interior partitions, utility
distribution, and mechanical equipment configurations. For animal research facilities, a grossing factor of at least 2.00+ is typical. A higher grossing factor could be possible depending on the choice of caging systems and circulation configurations.
top
B.4 Flexibility and Adaptability
Animal research facilities should be designed to maximize the animal holding capacity and related utility services. The animal facility should be flexible and adaptable to accommodate changes in function and protocols without having to make major changes to the facility. Spaces shall be designed to hold multiple species over time as protocols change. Individually planned or customized spaces are to be avoided.
B.4.1 Services and Systems: Utility systems within the animal research facility must be capable of providing all the services necessary for scientists to conduct their research and for the animal husbandry staff to properly care for the animals. It is equally important that provisions be made for utility services to accommodate unanticipated demands brought about by new technologies or changes in research protocols. A percentage of reserve capacity shall be designed into the primary building systems to accommodate increased animal densities. All components of the utility systems shall be planned and designed to allow all required access, maintenance, and repairs without entering the animal holding or procedure rooms. Maintenance spaces should be configured so they can be expanded without displacing animal research functions whenever possible. See Animal Research Facilities, Section: Design Criteria, Mechanical, for details on design of animal facility heating, ventilation, and air conditioning (HVAC) systems.
B.4.2 Expansion/Renovation Considerations: Vertical and horizontal expansion of an animal research facility shall be considered during the planning phase. It must be possible to construct any expansion with minimal interference to the operation of the facility and the least disturbance to the animal population. It is important for planning for expansion to be compatible with existing utility systems.
B.4.3 Connection of Utilities to Animal Facility Space: Utility services must be distributed to each individual space. The connection point of each service should be in a uniform position relative to the space and detailed to provide simple extension into the space without disruption of adjacent modules. These services may run in interstitial space, allowing animal holding or procedure space to change without increasing or upgrading the
capacity or location of central infrastructure systems. Changes would be primarily to terminal systems (i.e., piping and power connections to apparatus and equipment within the space).
B.4.4 Services and Systems Distribution Concepts: HVAC units serving animal facilities shall be designed with parallel HVAC system arrangements and or with standby equipment with capability to ensure continuous operation during equipment failure and scheduled maintenance outages. It is acceptable to have a common air intake system for both animal holding and other parts of the building. The animal area exhaust system must be
independent of the non-animal parts of the building.
Utilities and services, including communication and information systems, shall be organized into specific zones, both horizontally and vertically, to provide distribution of systems and services that can be extended to each animal holding and procedure module. The choice of design and location of the utility distribution system(s) is a product of utility function, costeffectiveness, and ease of access for maintenance, future services, and remodeling during the life of the animal research facility. At a minimum, a percentage of the holding and procedure rooms shall be designed for interchangeability of use. The percentage and locations of rooms with drains should be determined during programming.
B.4.4.1 Special Considerations for the Connection of Utilities to Animal Facility Space: The architect/engineer (A/E) shall make the consultants aware of the following special considerations for the connection of utilities to animal facility modules or space.
- Small-animal holding rooms other than isolation cubicles should each have a sink or the capability of easy installation of a sink if a need should present itself. Isolation cubicles are not required to have a sink, but a sink should be located in an adjacent procedure location/room. In all situations hand-washing sinks should be convenient to all holding locations. Large-animal holding rooms should have a sink outside the holding room area or suite.
- Farm animal holding rooms and aquatic tank rooms require floor drains. Most animal rooms for other species at the NIH are not hosed down, so drains should be avoided except in areas that may be converted in the future to hold aquatic or large-animal species. When floor drains are present, consideration should be given to the appropriate size of the drain lines, maintenance of the drain traps, drain caps and flush systems, and floor slopes to drains.
- The type of animal watering system should be determined (automatic or bottled) during programming. If automatic watering is not desired at the onset, consideration should be given to designing a system that can accommodate a percentage of automatic watering for possible future needs. Consideration should be given to the quality of water required. In some situations, highly purified water such as reverse osmosis (RO) may be required. In many cases there is an additional requirement for the treatment of the water prior to distribution (i.e., chlorination, acidification, or neutralization). Remote monitoring of the water treatment process is required. In order to accommodate water treatment concerns, appropriate equipment, piping, and plumbing systems must be considered.
- Consideration must be given to steam connections, clean steam, and RO water with additional polishing systems in the cagewash and bottle-filling area of the facility. Clean steam connections will be required wherever there is an autoclave. The users should also be consulted as to the need for RO water in dishwashers, if required, and frequency of RO drops in procedure rooms.
- The racking/caging system for small animals should be determined as early as possible in the planning process in order to determine the type and number of duct connections. Each racking system may have a different type of connection that will affect the placement of the exhaust duct. Consideration must be given to ducts above the plenum and the location and length of the exhaust taps. In addition, some racking systems are now designed with dynamic local area network (LAN) line connections.
- Placement of electrical outlets and waterproof protective covering for the outlets should be carefully considered for all animal holding rooms. Electrical loads must be sufficient to accommodate all the needs of animal holding and procedure rooms. Ideally, electrical outlets servicing ventilated rack turbo units and other equipment should be located high enough to prevent draping electrical cords, which are a safety hazard.
- LAN line connections. Consideration must be given to the requirement of LAN connections within the animal holding room.
top
B.5 Planning Module
Modular planning techniques have traditionally been employed to provide for an adaptable facility. Modular planning schemes shall be used, to the maximum extent possible, for animal housing and procedure space. Modular planning is based on the concept of threedimensional units of space and services, which are used in a repetitive fashion for each type of function within the animal facility. The dimensions of the structural bay, both vertically and horizontally, must be carefully evaluated with respect to the laboratory planning module, mechanical distribution, and future expansion plans. The planning module must be developed on the basis of an evaluation of operations and protocols and the anticipated numbers and species of animals.
In animal facilities, the most common unit of space is the animal housing/holding room. Ideally, when planning a multifunction animal research building, the animal holding room modular size should be determined on the basis of cage or rack system size. This scheme may or may not be similar in size and configuration to the standard laboratory module. The width of the animal room is determined by the number and types of animals, the way in
which they are housed, whether by cage or rack, and the cleaning methodology that will be employed. Room length is determined on the basis of housing/caging options and minimum aisle width between racks but also must accommodate service space for sinks, cleaning equipment, change stations, and so on. The height of the animal room is primarily a function of the maximum rack height anticipated, including rack fans. There must also be enough space above the rack to provide uniform airflow distribution in the room.
Wherever possible, rooms shall be clustered to provide separate zones for small and large animals, taking into consideration the differences in rack dimensions, waste disposal requirements, acoustical and vibration requirements, caretaking requirements, investigators, protocols, disease status, and airflow requirements.
B.5.1 Animal Facility Holding, Procedure, and Support Module Variations: The length, width, and height of the animal facility modules are dependent on the intended use of the space. There may be a need to a have a variety of sizes of small-animal holding rooms with or without individual or shared anterooms. Animal holding and procedure suites are a combination of modules used for a specific research purpose. Within a suite, the rooms may be subdivided or positioned differently from the general layout of the animal facility. Other support spaces such as the cagewash or administrative areas are composed of multiple modules without wall divisions to accommodate large pieces of equipment or open office space.
B.5.2 Structural Bay Spacing: The structural loads of an animal facility are quite substantial because of the potential use of concrete masonry partitions and heavy equipment that is needed for the day-to-day operation of the facility. The most basic requirement of the structural system is that it not interfere with other systems or preclude future changes. Therefore, the spacing of both the vertical and horizontal components of the structural system must be coordinated with the room and corridor configuration and utility systems distribution.
top
B.6 Functional Relationships and Zoning of the Animal Research Facility
The zones in an animal research facility can be grouped into four categories that are further characterized as “clean” or “dirty.” Clean or dirty refers to the potential for the animal or material to transmit diseases to other animals from outside sources. For example, animals from an unapproved source are considered “dirty” until they have been evaluated for health status during a quarantine period. Barriers within the facility are “clean” and should receive only “clean” approved animals and materials; used cages are “dirty” and should not move into designated “clean” areas because they may be a source of contamination.
B.6.1 Public Zones: Public zones include public corridors and elevators, multi-use loading docks, supply rooms, laboratories outside barrier areas, and areas where staff wear street clothes. Public zones are categorized as “dirty” because there is no control of potential animal contaminants in these areas.
B.6.2 Transitional Zones: Transitional zones are defined as areas of movement between public areas and animal holding and procedure areas or between zones housing different animal species that could potentially transmit diseases between each other if they were in close contact. Transitional zones may include airlocks, gowning areas, locker rooms, feed and storage areas, and dedicated “clean” and “dirty” animal elevators.
B.6.3 Specific-Pathogen Free (SPF) Zones: SPF zones are areas where animals are free of defined diseases. The degree of SPF may vary in different parts of the facility just as the degree of “clean” and “dirty” may vary. The level of SPF and “clean”/“dirty” will be defined by the veterinarians and the users of the facility. Most housing/holding areas are located in the SPF zone. An exception to this occurs when “dirty” animals (nondefined disease status) are needed for the research. A separate housing area that contains isolation housing and/or has an airlock shall be provided for this purpose.
B.6.4 Contaminated Zones: Contaminated zones are areas where dead or infected/diseased animals are located or where “dirty” equipment is transported or stored. There are instances where conventional housing is required for “dirty” animals such as a quarantine room or an area of the facility specifically for research using “dirty” (non-SPF) animals. Circulation routes must be closely examined in these situations so as to minimize cross-contamination of SPF animals.
“Dirty” corridors are those used for moving soiled cages and materials to the “dirty” side of the cagewash facility. Rooms where necropsies or perfusions (terminal procedures) are performed are defined as “dirty.” A single corridor system can be managed so as to provide the desired degree of cleanliness and species separation defined by the facility program.
B.6.5 Zone Relationships: Within the animal research facility, the flow of materials, cages,
Figure B.6.5 Functional Relationship Diagram for an Animal Research Facility
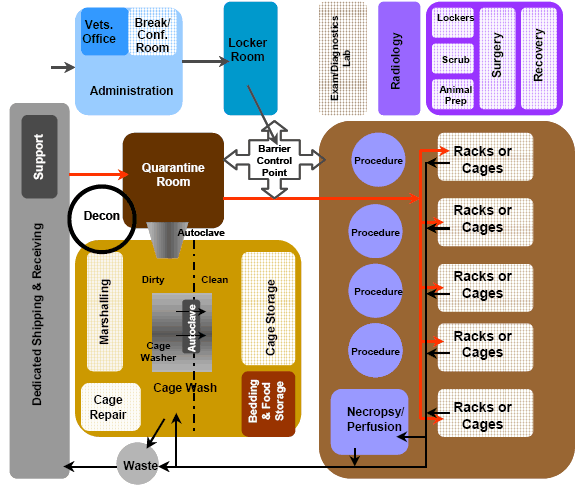
animals, and personnel must be accommodated in an efficient and economical manner. Adjacencies shall be planned to maximize operational affinities and minimize travel distances. Relationships among deliveries, animal receiving, food and bedding, quarantine, housing, procedure rooms, storage, cagewash, staff locker rooms, and administration spaces must be effectively planned. It is also essential that designs consider adjacencies based on the variety of species that are anticipated for the animal research facility.
top
B.7 Circulation of People, Animals, and Materials
Circulation space is a critical factor in controlling contaminants and enhancing operations and procedures within the animal research facility. Planning of circulation focuses on the movement of cages and racks in the facility, since this is the most intensive use of this space. Most importantly, during the planning phase, the design team decides to what extent the corridor system helps manage the potential for contamination and to what extent management dictates certain protocols of time and direction of movement. Personnel, equipment, and supplies should move from areas of least contamination to areas of greater contamination. Movement of personnel, equipment, and supplies should be planned to minimize the potential for contamination of cleaner areas. Consideration should be given to the equipment and areas required to permit recirculation of supplies, personnel, and
equipment. For example, autoclaves located near the “dirty” cagewash permits the recirculation of contaminated caging back into the cagewash area, and convenient location of locker rooms and shower facilities in many cases permits personnel to move from “dirtier” areas back into cleaner situations.
B.7.1 Horizontal Circulation Corridors: Commonly accepted circulation systems include a single corridor, a dual “clean” and “dirty” corridor, or a single corridor with unidirectional flow. The NIH does not use the dual-corridor system when planning new facilities.
B.7.1.1 Single-Corridor System: In a single-corridor scheme, traffic flow is in both directions between the animal holding room and the cagewash area. The most significant advantage of a single-corridor system is its efficiency of space utilization. The disadvantage is the potential for cross-contamination in the corridor when “clean” and “dirty” cages share space. Congestion caused by moving animals, cages, and supplies through a single
corridor is also problematic. However, contact between “clean” and “dirty” materials can be minimized by carefully scheduling pickups and deliveries, covering cages when moving them, and using a unidirectional circulation system. With this management technique, congestion and contamination can be minimized. Single-corridor systems shall be equipped with appropriately placed air locks and doors to maintain the desired level of facility sanitation and security. Placement of air locks must be discussed with users. Doors should have controlled access where necessary.
B.7.1.2 Dual-Corridor System: Contamination control is the primary rationale for choosing a dual-corridor system. The dual corridor system has animal holding rooms leading to two separate corridors that are dedicated “clean” and “dirty” corridors for the movement of cages. The flow of cages is unidirectional and may involve two single-loaded corridors in a small facility or one double-loaded and two single-loaded corridors in a larger facility. Dual corridors are not an efficient use of space and will increase the gross-to-net ratio.
B.7.1.3 Corridor Width: Corridor width should be dependent on the flow of traffic within the animal facility and the amount of storage that will be available in or near the facility. The Guide recommends a corridor width of 1 825-2 450 mm, but 3 050-3 650 mm-wide corridors allow for more flexibility in circulation in larger facilities. Two animal cage racks must be able to pass each other comfortably in the corridor. Sufficient storage must be designed in or near the facility so that equipment does not have to be stored in the corridors. Marshalling alcoves for racks and carts should be provided so that corridors are kept free of this equipment.
B.7.2 Vertical Circulation Elevators: In multilevel facilities, dedicated “clean” and “dirty” animal elevators are required. The elevator for transporting “clean” material should be located near the “clean” side of the cagewash area, while the elevator used for “dirty” material should be in close proximity to the “dirty” side of the cagewash area. The elevator size and location must accommodate the volume of materials to be handled in the cagewash, animal and material receiving, and waste removal areas. Elevators that will be used for transport of animals and animal facility equipment must be constructed of highly durable and cleanable materials. The elevator cab floor material must be of the same material as the floor in the animal facility. The elevator car interior should have guardrails at appropriate heights for the typical racks and carts that will be used in the facility. Elevator doors must be of sufficient height to accommodate the tallest racks that will be used in the facility. Consideration should be given to an elevator door width that can accommodate at least two racks side by side.
At least one elevator should have the capacity to handle extremely heavy loads if, for instance, an irradiator is planned in the facility on a level below the loading dock level of the building. There should be adequate redundancy in the number of elevators to handle freight, staff, and animals in the case of an equipment breakdown. This can be accomplished by locating the “clean” and “dirty” elevators near each other so that they can serve as backups to each other.
top
B.8 Security
See General Design Guidelines, Section: Security, for general design guidance. The objective of security in an animal research facility is to ensure the safety of the animals, staff, equipment, and data. Animal research facility users must be aware of the levels of security at the site, building, animal research facility, and room levels. At NIH-owned or - leased facilities, the site is the first level of security. The site may be open to the public, or it
may have controlled access depending on the location. The second level of security is the building. Access to the building must be managed. Air intakes and any central utilities must be safeguarded from intruders. The third level of security is the access to the animal research facility. Administrative staff, research and veterinary staff, maintenance staff, and vendors will require access to the animal research facility. A central reception area is recommended as a gatekeeper entrance area to the animal research facility. Security features must also be provided for the loading docks and service entries for the animal
facility. Finally, the fourth level of security is the specific animal rooms, containment suites, surgical suites, pharmacy, or other areas within the animal facility with a higher level of controlled access and surveillance. An internal facility system designed to limit/control access to animal holding rooms and other areas has proven to be a useful facility management tool.
top
B.9 Loading Docks
See General Design Guidelines, Section: Site/Civil, for general loading dock design guidelines. The loading dock that services a building with an animal facility should include a dedicated bay for animal and material receiving and waste removal. The animal care loading dock must be viewed as an extension of the animal care facility. Excluding pests and creating conditions that promote proper sanitation, at this location, are imperative to
maintaining a pest-free facility that meets or exceeds AAALAC guidelines. To achieve these desired goals of pest exclusion and good sanitation, the dock facility must be properly sited, constructed of durable, cleanable materials, sized to meet current and future program needs, flexible in use, and effective as a barrier between the outside and the “clean” environment of the animal care facility. The animal-receiving loading dock should include:
- A dedicated animal facility bay that is visually protected for security. The dock must be physically segregated form other dock space and dock functions. This includes vehicle docking and material/supplies staging space.
- A receiving vestibule that is temperature and humidity controlled to protect valuable research animals. Overhead doors should be fitted with proper sweeps, gaskets, and brushes to exclude insects and rodent pests around the perimeter of the entire door. These doors and doorframes must provide an effective seal, when closed, to exclude insect and rodent pests. The loading dock doors should be equipped with air curtains or
other similar devices to exclude flying insects and to create a dust and dirt barrier when the receiving or personnel doors are opened.
- A dedicated route of transportation into the animal facility if possible.
- A large pass-through autoclave if bedding is to be sterilized at the loading dock.
- An area to decontaminate the animal containers before they enter the animal facility. The decontamination area can be at the loading dock or at the point of entry to the animal facility. The interior surfaces should be covered with materials that facilitate proper sanitation and ease of cleaning. These materials must be durable enough to withstand regular cleaning and disinfection. Facilities must be available for loading dock washdown and cleanup. Floor drains should not be designed into the receiving area of the loading dock.
- A cold storage room for animal carcasses.
- The dock entry points (e.g., materials receiving or personnel) must be isolated from solid waste compacting, handling, and storage operations. Solid waste operation can be attractive to pest species that are invasive to the facility.
- Recycling containers should not be sited on or near an animal facility loading dock. Waste should not be staged for removal inside the receiving area of the loading dock.
- For guidance on disposal of radioactive medical pathological waste from an animal facility, see Animal Research Facilities, Section: Space Descriptions, Animal Research Facility Support.
- There should be no exposed conduit, piping, ledges, wall-mounted lights, and so on. These provide loafing and nesting sites for nuisance birds and are difficult to clean.
- Wall, corner, and door guards should be of a type used inside the animal care facility (i.e., stainless steel, caulked and sealed at installation).
- Caulking and sealing of all floor, wall, and ceiling penetrations; wall placards; switch plates; outlet covers; and so on are required. Caulking and sealing should entail the use of the same products and procedures as those used inside the animal care facility.
- Electrical service should be provided on all walls of the receiving area and the elevator lobby to power electric light traps for pest exclusion.
- Lighting should be indirect to the loading dock to reduce attraction of flying insects. Do not use wall-mounted lighting. Do not install lights directly above receiving or personnel doors.
- Provide a dedicated animal loading dock manager’s office.
- Provide chemical storage.
- Provide gas cylinder storage.