
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-209 - 2,3,7,8-Tetrachlorodebenzo-p-Dioxin
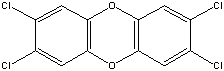
| Chemical Formula: C12H4Cl4O2 | - | 3D Structure* |
|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | ||
A carcinogenesis bioassay of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a contaminant in several phenoxy herbicides, was conducted by administering TCDD by gavage to Osborne-Mendel rats and B6C3F1 mice for 104 weeks.
Fifty rats and mice of each sex were given TCDD suspended in a vehicle of 9:1 corn oil-acetone 2 days per week for 104 weeks at doses of 0.01, 0.05, or 0.5 µg/kg/wk for rats and male mice and 0.04, 0.2, or 2.0 µg/kg/wk for female mice. Seventy-five rats and 75 mice of each sex served as vehicle controls. One untreated control group containing 25 rats and 25 mice of each sex was present in the TCDD treatment room, and one untreated control group containing 25 rats and 25 mice of each sex was present in the vehicle-control room. All surviving animals were killed at 105 to 108 weeks.
In rats, a dose-related depression in mean body weight gain was observed in the males after week 55 of the bioassay and in the females after week 45. In mice, the mean body weight gain in the dosed groups was comparable to that of the vehicle-control groups.
In male rats, increased incidences of follicular-cell adenomas in the thyroid were dose related and were significantly (P=0.001) higher in the high-dose group than in the vehicle controls (1/69, 1%; 5/48, 10%; 6/50, 12%; 10/50, 20%). Similarly in the female rats, an increase (though not statistically significant) was seen in the high-dose group (3/73, 4%; 2/45, 4%; 1/49, 2%; 6/47, 13%).
In female rats, the incidence of neoplastic nodules of the liver in the high-dose group was significantly (P=0.006) higher than that in the vehicle control group (5/75, 7%; 1/49, 2%; 3/50, 6%; 12/49, 24%).
In male and female mice, incidences of hepatocellular carcinomas were dose related and the incidences in the high-dose groups were significantly (P=0.002 and 0.014, respectively) higher than those in the corresponding vehicle control groups (males: 8/73, 11%; 9/49, 18%; 9/49, 16%; 17/50, 34%; females: 1/73, 1%; 2/50, 4%; 2/48, 4%; 6/47, 13%).
In female mice, follicular-cell adenomas in the thyroid occurred at dose-related incidences, and were significantly (P=0.009) higher in the high-dose groups than those in the vehicle controls (0/69, 0%; 3/50, 6%; 1/47, 2%; 5/46, 11%).
Increased incidences of toxic hepatitis related to the administration of the test chemical were detected among high-dose rats and high-dose mice of each sex.
Under the conditions of this bioassay, 2,3,7,8-tetrachlorodibenzo-p-dioxin was carcinogenic for Osborne-Mendel rats, including follicular-cell thyroid adenomas in males and neoplastic nodules of the liver in females. TCDD was also carcinogenic for B6C3F1 mice, including hepatocellular carcinomas in male and females and follicular-cell thyroid adenomas in females.
Levels of Evidence of Carcinogenicity: | ||
| Male Rats: | Positive | |
| Female Rats: | Positive | |
| Male Mice: | Positive | |
| Female Mice: | Positive | |
Synonyms: 2,3,7,8-TCDD; TCDD
Note: 2,3,7,8-Tetrachlorodibenzo-p-dioxin was previously tested in Swiss-Webster mice administered dermally (See TR-201, reported 1982).
Report Date: February 1982
Target Organs & Incidences from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 14, 2004
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.