 |
|
|
July
2006
|
 |
|
|
| |
 |
|

Researchers Gain Insight Into Why Brain Areas Fail
To Work Together in Autism, July 12, 2006
Dopamine Drug Leads to New Neurons and Recovery of
Function in Rat Model of Parkinson's Disease, July
4, 2006
Culprit in Grapefruit Juice Drug Interaction
Identified, June 23, 2006
Pandemic Flu Information
Institute and center news links
NIGMS Feedback Loop
Inside NIDCD
NIH Radio archives available for listening and
downloading
NIH Radio is a 24-hour audio service designed
to provide radio stations with the latest
information about NIH research findings,
highlights of press conferences and health
campaigns.
This service features weekly, short, health-related
news stories distributed to radio stations
throughout the United States. Archives of various
stories are available through Web-based written
transcripts, as downloadable MP3 files, and
through streaming RealPlayer format.
Recent archived radio reports
include:
NHLBI Offers Updated Guide with Practical
Advice for Lowering High Blood Pressure Through
Diet, July 2006
State of the Science Conference Discusses
Multivitamins and Mineral Supplements, May 2006
Research Program Hopes to Improve Survival Rate
for Cardiac Arrest and Traumatic Injury, April
2006
 Back
to top Back
to top
|
|
|
|

|
|
NRSA F32 Application
Streamlined Review Formally Announced
The
May issue of the NIH Extramural Nexus
highlighted plans to streamline review of Ruth L.
Kirschstein National Research Service Awards
(NRSA) Postdoctoral Fellowship Applications (F32).
NIH now has published the
Guide Notice, announcing to the
applicant community the streamlined review
process.
Opportunity for Funding Extension and/or One-Time Supplements for Hurricane Katrina-Affected Grants
In an effort to assist grantees located in New Orleans with active NIH grants who have persevered through the challenges brought by Hurricane Katrina, the NIH will provide the opportunity for investigators and institutions to request up to a one-year funded extension at the same funding level as the current final year of the grant and/or one-time administrative supplements of up to $50,000 in direct costs. This applies to single project research project grants active at the time of the storm that remain in New Orleans.
Complete information, including details of
documents to submit, deadlines and contact
information are available in the
Guide
notice.
NIH to Seek Public Input on Changes Being Considered on Use of Appendixes in Grant Applications
NIH is evaluating guidelines for grant application appendixes in an effort to streamline the
grant application and review processes. NIH soon
will issue a Request for Information (RFI) in the
Federal Register that seeks input from applicants, reviewers and other members of the research community regarding the utility of appendix materials in the grant submission, review and management process. Look for the notice in early August.
 Back
to top Back
to top
|
|
| |

|
|
|
|
|
 |
|
|
| |
 |
|
COMMUNICATE WITH
THE
NIH EXTRAMURAL NEXUS—WE WANT TO HEAR FROM
YOU
Feedback (to the Editor) from recipients and
subscribers of the NIH Extramural Nexus is
vital. Your comments, questions, and suggestions for
topics will enable Nexus editorial staff to
deliver appropriate content to the extramural
community.
 Back
to top Back
to top
|
|
| |
|
Printer-Friendly Version
(Adobe Acrobat Reader Required) |
|
|
|
NEWS FROM THE DIRECTOR OF OER:
NIH’s Partnership with the Extramural
Community—Reporting
Conflict of Interest |
|
 Dear
Extramural Community: Dear
Extramural Community:
NIH’s relationship with the extramural
community is a partnership in which the NIH,
investigators and their academic and research
institutions, and private industry each play
unique and essential roles in the quest to
discover new knowledge to address the biomedical
health needs of the nation. Our partnership
benefits from the shared and unique perspectives
and interests that each of us brings to the table.
In this dynamic and complex environment, conflict
of interest is inevitable. But, we share the
common goal of identifying and managing such
conflicts so that they in no way diminish our
ability to carry out our missions effectively or
endanger the public’s trust in us.
I do not mean to imply that an investigator
with a conflict of interest, particularly a
financial one, cannot engage in NIH-sponsored
research. Depending on the circumstances, the
conflict may be, as described by Federal
regulation, managed, reduced, or eliminated by the
institution, allowing the investigator to conduct
the research. However, regardless of the outcome,
after the identification of a financial conflict,
both the existence of the conflict and whether it
has been managed, reduced, or eliminated must be
reported to the NIH, to ensure integrity and
objectivity in research, maintain public trust,
and comply with Federal regulation.
NIH and institutions that receive extramural
awards are aware of the potential for substantial
negative impact that unidentified or unmanaged
financial conflicts of interest can have on the
integrity of the research they conduct and on
their reputation. We also are aware that grantee
institutions sometimes face considerable
challenges in implementing and complying with the
requirements of the Federal regulation.
Consequently, last fall NIH began a series of site
visits to determine whether grantee institutions
have correctly implemented Federal financial
conflict of interest regulations and if reporting
requirements are being met. We have completed 12
of these site visits and are planning several more
before the end of the calendar year.
These site visits have provided NIH with
valuable insight into those parts of the
regulation that present grantee institutions with
the greatest implementation challenges. We are
working with institutions to address these
problems. In addition, we plan to share our
observations publicly so that the entire
extramural community can benefit from the
experiences of those site-visited.
Below, you will find a summary of the
Financial Conflict of Interest (FCOI)
Regulation, which explains the requirements of
the regulation as well as the responsibilities of
grantee institutions and the NIH. I hope that you
will find the information helpful. As always,
please feel free to get in touch with me or with
OER staff if you have questions or comments.
NIH recognizes that significant resources are
invested by institutions to identify, manage,
reduce, or eliminate financial conflicts of
interest. I can assure you that OER staff will
continue its efforts to support institutions by
providing clear guidance and consultation. We must
ensure that financial conflict of interest is
disclosed, reported, and managed in a way that is
appropriate for the proposed use of taxpayer funds
and preserves the public’s trust. NIH truly values
its partnership with the extramural community. It
has served us all well for many years, and I look
forward to our continuing work together to address
this and any other difficult challenge that we may
encounter.
Summary of the Financial Conflict of Interest
Regulation:
 |
Investigator is broadly defined as the Principal Investigator (PI) and
any other person who is responsible for the design, conduct, or reporting
of research funded by PHS or proposed for such funding. The definition includes
the investigator’s spouse and dependent children. |
 |
At the time of application, an investigator, which by definition includes
any spouse and dependent children, is required to submit to the institution a financial
disclosure (a listing of all significant financial interests). |
 |
Financial disclosures must be updated during the period of award,
either annually or as new reportable interests are obtained. |
 |
A significant financial interest is defined as anything of monetary value,
with exceptions (see
42 CFR Section 50.603 for a full definition of significant financial
interests). The minimum threshold established in the regulation for
investigator's significant financial interests is $10,000 in value or a five-percent ownership in a single entity. |
 |
Prior to the expenditure of funds under an award, the institution is
required to report to the NIH awarding component the existence of a conflict of
interest, but not the nature of the interest or other details, and assure that
the interest has been managed, reduced, or eliminated. Conflicting interests
that are subsequently identified must also be reported within 60 days of
identification.
|
 |
As necessary, NIH may request additional information about reported
conflicts. |
 |
Awardees are required to ensure that subcontractors and subgrantees either
comply with the institution’s conflict of interest policy, or assure that they will enable the
collaborating institution to comply.
|
— Norka Ruiz Bravo, Ph.D. -
Director, OER and NIH Deputy Director for
Extramural Research
 Back
to top Back
to top
|
|
NIH Roadmap
for Medical Research:
NIH
Outlines Process for Idea Solicitation and
Initiative Selection |
|

In 2002, NIH Director Dr. Elias Zerhouni
conducted a series of discussions with NIH
advisors and the scientific community about ways
to improve and accelerate biomedical research.
These discussions led to the creation of the
NIH Roadmap for Medical Research. Roadmap is a
process designed to increase synergy across all of
NIH, enabling NIH to address proactively emerging
scientific needs and opportunities; fund high
risk, high impact science; and, incubate and
launch pilot efforts that have the potential to
transform science. Because Roadmap is intended to
evolve constantly and adapt to the realities of
science, projects within Roadmap are limited to a
maximum of 10 years of support.
Building on the success of the first version of
the Roadmap for Medical Research, NIH is beginning
a process to identify ideas for a new cohort of
Roadmap initiatives to be funded within the
existing Roadmap budget in fiscal year 2008. Funds
for these new initiatives will become available as
current Roadmap initiatives conclude, are adjusted
in scale, or their initial funding plans are
modified.
In the first part of the process to identify new
initiatives, ideas will be solicited through
meetings with scientists from around the nation
that represent various disciplines and areas of
investigation. These meetings will take place
during the summer and early fall of this year and
will help senior NIH staff identify cross-cutting
challenges in biomedical research that meet the
Criteria for Roadmap Initiatives shown in the box
below.
| The overarching goal of all Roadmap initiatives is to accelerate the
discovery and translation of scientific knowledge into public health benefits.
Roadmap is conceived of as a five to ten year ‘incubator space’ for NIH
initiatives that meet all of the following criteria: |
 |
Is the proposed initiative truly
transforming–could it dramatically
affect how biomedical and/or
behavioral research is conducted
over the next decade? |
 |
Will the outcomes from the
proposed initiatives synergistically
promote and advance the individual
missions of NIH ICs to benefit
health? |
 |
Does the proposed initiative
require participation from NIH as a
whole and/or does it address an
area(s) of science that does not
clearly fall within the mission of
any one IC or OD program office? |
 |
Is the proposed initiative
something that no other entity is
likely or able to do, and is there a
public health benefit to having the
results of the research in the
public domain? |
|
|
NIH Institutes, Centers and Offices will be
given an opportunity to develop Roadmap ideas this
summer. Idea descriptions from both activities
will be posted when the NIH launches a web-based
Request for Information (RFI) soliciting a broad
range of input and ideas from both science and lay
communities this fall. The RFI will provide an
opportunity for respondents to comment on the
ideas already suggested and/or submit new ideas.
All idea nominations will be screened to make
sure that they are consistent with the Criteria for
Roadmap Initiatives, then clustered by topic area
and summarized for further review and
prioritization by NIH IC Directors. Implementation
plans will be developed for high priority proposals
in the following months and a final prioritization
by IC Directors will be sent to the NIH Director,
who will consult with the Advisory Council to the
Director (ACD) prior to making funding decisions
next spring.
Once selected, new Roadmap initiatives will be
formally assigned a lead IC and a trans-NIH
Project Team. Project Teams will generate
solicitations for the types of projects required
to fulfill the initiative’s aims. Applications
will be received, reviewed, approved and funded
beginning in FY2008.
The RFI and summary information from each phase of
the idea nomination process will be posted as it
becomes available on the
Office of Portfolio Analysis and Strategic
Initiatives/Roadmap Web site. Additional
information on this process will appear in the
NIH Guide for Grants and Contracts.
 Back
to top Back
to top |
|
ELECTRONIC Submission
News: |
|
Updated Forms
For eSubmission
Are Here:
Grants.gov and NIH Update SF424 (R&R) and
Agency-Specific Forms |
|
 Grants.gov
and NIH recently updated the standard form SF424
(R&R) and agency-specific PHS 398 electronic grant
application form components used for electronic
submission of grant applications. The changes
provide greater standardization among application
forms and incorporate new policy requirements. All
new NIH Funding Opportunity Announcements (FOAs)
will use the revised application form. Over the next
few months, NIH will update existing FOAs that use
the older forms to reference the new application. Grants.gov
and NIH recently updated the standard form SF424
(R&R) and agency-specific PHS 398 electronic grant
application form components used for electronic
submission of grant applications. The changes
provide greater standardization among application
forms and incorporate new policy requirements. All
new NIH Funding Opportunity Announcements (FOAs)
will use the revised application form. Over the next
few months, NIH will update existing FOAs that use
the older forms to reference the new application.
To summarize, we have updated sections and
components of the grant application forms. New grant
opportunity announcements will reference the revised
application, and old announcements will be revised
to reflect the new application form.
We will maintain and support the old and new
application packages and their associated
application instructions until we are sure that all
applications approved to use the old form set have
been received and processed.
What the new application form means for applicants:
 |
New FOAs will be posted citing the revised application packages. No special action needs
to be taken. |
 |
FOAs issued before June 15, 2006 that expire on or before December 31, 2006,
will be allowed to close naturally without issuing any revised application form. No special action needs to be taken. |
 |
For older FOAs with multiple future receipt dates, NIH will update the FOA
to expire the old application form and reflect the revised application. Once
the new application form is in place for a particular FOA, the old application will
no longer be accepted by Grants.gov. Any application “in the works” will need to
be moved to the new form package (i.e., download new forms and cut-and-paste
content from one form to the other). Older FOAs will be updated as follows: |
| |
 |
New forms will be in place by July 18
for the following grant programs: R03, R15,
R21, R21/33, R33 and R34 |
 |
New forms will be in place by September
15 for the following grant programs: R41,
R42, R43, R44, R13/U13 |
|
 |
To differentiate between the old and new application, the new application will be
labeled Version 2 Forms in the Competition ID field. |
 |
There will be a short period of time when both the old and new application packages
will be available to download. Whenever there is a choice, the package labeled
Version 2 Forms in the Competition ID field should be used. |
 |
If your business office provides versions of the application packages to Principal Investigators
that are pre-populated with your organization's information, be sure your
application package templates use the revised application package. |
 |
The existing and revised application guides will be labeled Version 1 and
Version 2 on the cover page and within the footers. The FOA will
continue to link you to the appropriate application guide. |
The following table shows when to use the new forms:
Posting/Release
Date of FOA |
Grant
Mechanism |
Use New
Forms |
Before
June 15, 2006* |
R03, R15, R21, R21/33,
R33 and R34 |
After July 18 |
Before
June 15, 2006* |
SBIR/STTR
(R41, R42, R43, R44),
R13/U13 |
After Sept. 15 |
On or after
June 15, 2006 |
All application packages
will contain the new forms |
√ |
* Only FOAs that expire after December 31, 2006
For more information, visit the
eSubmission Frequently Asked Questions Web page.
|
ALERTS! |
|
Issue with Version 2 Application Package |
 An
issue with the PureEdge™ version of the Research & Related
Senior/Key Person Profile component included in the Version 2 packages has been
identified. The
section of the PureEdge form that allows attachments for Additional Senior/Key
Person Profiles, Biographical Sketches and Current and Pending Support is
missing. Without this section applications are limited to eight Senior Key
Persons. NIH will be issuing a notice in the NIH Guide for Grants and Contracts
providing a workaround for applicants to use when more than eight Senior Key
Persons need to be listed on an application. This workaround will remain in
place until the Research & Related Senior/Key Person Profile component is
corrected by Grants.gov. An
issue with the PureEdge™ version of the Research & Related
Senior/Key Person Profile component included in the Version 2 packages has been
identified. The
section of the PureEdge form that allows attachments for Additional Senior/Key
Person Profiles, Biographical Sketches and Current and Pending Support is
missing. Without this section applications are limited to eight Senior Key
Persons. NIH will be issuing a notice in the NIH Guide for Grants and Contracts
providing a workaround for applicants to use when more than eight Senior Key
Persons need to be listed on an application. This workaround will remain in
place until the Research & Related Senior/Key Person Profile component is
corrected by Grants.gov. |
|
Version 2 Application Form Automated
Emails |
|
In
an effort to keep Applicants informed of changes
to Funding Opportunity Announcements (FOAs),
Grants.gov provides an automated email process to
subscribers. The new Version 2 application package
triggered recent emails that have caused some
confusion for Applicants.
For more information regarding the automated
emails visit the
eSubmission News and Updates Web page. |
|
 Back
to top Back
to top
Did You Check Your Assembled Application?
 Applying
for an NIH grant opportunity, you filled in all the application fields,
included the required attachments and forwarded your application package to your Authorized Organization
Representative (AOR) in time to meet the receipt deadline. But, your job does
not stop there. Did you track the status in the eRA Commons? Did you
check your assembled application? Applying
for an NIH grant opportunity, you filled in all the application fields,
included the required attachments and forwarded your application package to your Authorized Organization
Representative (AOR) in time to meet the receipt deadline. But, your job does
not stop there. Did you track the status in the eRA Commons? Did you
check your assembled application? We continue to receive calls, well after the
two-day viewing window has elapsed and the
application has moved forward to the Division of
Receipt and Referral, from panicked PIs who have
just noticed serious issues with their
applications. One applicant attached the NIH
application guide to their application by mistake!
Another applicant had a an upside down PDF image
in the assembled application. Unfortunately, after
the two-day viewing period and once the
application has moved forward to the Division of
Receipt and Referral, Help Desk staff no longer
can assist with changes to the application.
Nobody cares more about your grant application than you do. Take advantage of
the two-day viewing window to give your application that final check—then
relax knowing your part of the submission process is truly complete.
Check out our new Frequently Asked Questions on Checking Assembled Applications.
 Back
to top Back
to top
NIH Easily Handles June 1 Application Submission Date
 The
NIH has received over 9,000 unique research grant
applications electronically through Grants.gov
since launching its electronic submission program
in August 2005. The June/July submission round
marked a significant milestone in NIH’s
eSubmission program with the transition of the
Small Grant (R03), Exploratory / Developmental
Research (R21/R33) and Clinical Trial Planning
(R34) grant mechanisms to electronic submission.
NIH received applications submitted to Grants.gov
using PureEdge™ forms, from institutions that have
developed system-to-system solutions, and from
commercial service providers. It was not only the
largest transition to date; it was the smoothest
for the applicant community! NIH received over
4,000 applications for the June/July transition
round, which is right in the expected range. The
NIH has received over 9,000 unique research grant
applications electronically through Grants.gov
since launching its electronic submission program
in August 2005. The June/July submission round
marked a significant milestone in NIH’s
eSubmission program with the transition of the
Small Grant (R03), Exploratory / Developmental
Research (R21/R33) and Clinical Trial Planning
(R34) grant mechanisms to electronic submission.
NIH received applications submitted to Grants.gov
using PureEdge™ forms, from institutions that have
developed system-to-system solutions, and from
commercial service providers. It was not only the
largest transition to date; it was the smoothest
for the applicant community! NIH received over
4,000 applications for the June/July transition
round, which is right in the expected range.
eRA Commons processing ability and software
continue to improve. System improvements,
aggressive education efforts and many
well-prepared Offices of Sponsored Research
dramatically reduced the number of submission
attempts per successful submission and the number
of support calls to the eRA Commons Help Desk.
Many people were looking at the June 1 submission
date as an early indication of what to expect with
the transition of the traditional Research Project
Grant Program (R01) applications in February 2007.
“Applications are getting in the door and ready
for processing by the Division of Receipt and
Referral much more quickly than for previous
eSubmission receipt dates. We now see very few
system issues that require NIH intervention to
process applications. The Help Desk is better able
to handle call volume. Communications efforts are
paying off and many applications now are going
through on the first try. We have a long way to go
and some tough challenges ahead, but the
continuous improvements are encouraging. We are
looking forward to the February R01 transition,”
commented Megan Columbus, NIH Program Manager for
the Electronic Submission of Grant Applications.
 Back
to top Back
to top |
Multiple
Principal Investigator Update:
Applications
Indicate that the Multi-PI Option is Attractive |
|
 After
decades of associating a single Principal
Investigator (PI) with each grant, on May 11, the
NIH received its first Multiple PI applications in
response to a Request for Applications (RFA). A
recent NIDDK RFA designed to stimulate research on
the
Obese and Diabetic Intrauterine Environment
permitted Multi-PI applications under the test
initiative. Nearly 30 percent of the applicants
selected the multiple PI option. Almost 50 percent
of the applications received in response to the
NCI's RFA for
Small Animal Imaging Resource Program displayed
more than one PI. A smaller proportion of the
applications for the
NLM Research Grants in Biomedical and Bioinformatics
program, received on June 1, involved multiple PIs. The
pilot's current level of response in the Multiple-PI
option indicates considerable interest in selected research areas. The receipt and processing of the first
multiple-PI applications are important milestones
in the implementation of what is anticipated to be a
broadly transforming
Roadmap activity. After
decades of associating a single Principal
Investigator (PI) with each grant, on May 11, the
NIH received its first Multiple PI applications in
response to a Request for Applications (RFA). A
recent NIDDK RFA designed to stimulate research on
the
Obese and Diabetic Intrauterine Environment
permitted Multi-PI applications under the test
initiative. Nearly 30 percent of the applicants
selected the multiple PI option. Almost 50 percent
of the applications received in response to the
NCI's RFA for
Small Animal Imaging Resource Program displayed
more than one PI. A smaller proportion of the
applications for the
NLM Research Grants in Biomedical and Bioinformatics
program, received on June 1, involved multiple PIs. The
pilot's current level of response in the Multiple-PI
option indicates considerable interest in selected research areas. The receipt and processing of the first
multiple-PI applications are important milestones
in the implementation of what is anticipated to be a
broadly transforming
Roadmap activity.
The Multiple-PI option is on track
to be available for nearly all research project grant
opportunities starting with the February 1, 2007,
receipt date. More complex grant opportunities will
allow a Multiple-PI option as they
transition to electronic submission format. Updates on
the pilot will be posted on the
Multiple PI Web site.
 Back
to top Back
to top |
|
WHO IS A NEW
INVESTIGATOR?
An Update |
|

The May 2006 issue of the NIH Extramural Nexus, included an
article on the NIH definition of a new
investigator.
Since then, NIH has received the following question on this issue:
Question:
I became the PI on an NIH Traditional Research Project Grant (R01) when my
supervisor retired. Will I be eligible to compete as a new investigator when
this grant comes up for competing renewal?
Answer:
No. The definition of New Investigator (see
PHS 398 Instructions; Section I: Preparing Your Application, Part C:
Specific Instructions, Item 1: Face Page) is someone who has not previously
served as a PI on a PHS-supported research grant (with some exceptions, as
detailed in the definition). This particular scientist has been fortunate to
serve as PI even though he did not compete. Based on the definition, however, he
is no longer eligible to compete as a new investigator.
Additional information is available at the
New Investigators Program Web site.
 Back
to top Back
to top |
|
Interactive
State and Congressional District Award Data Now
Available |
|
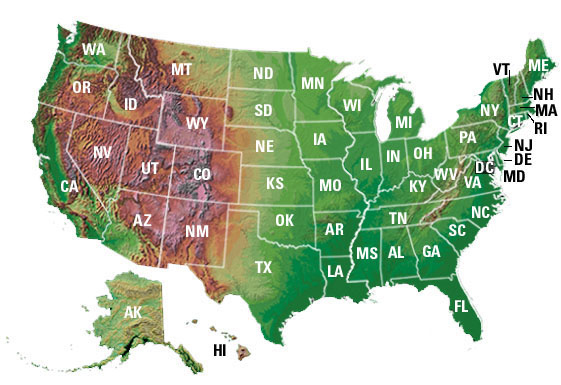 NIH's
Award Trends Web site now lists current and
historical data on NIH awards to states,
congressional districts, universities and other
institutions. That information is now available
through an interactive interface and through
the
NIH Research Results for the Public Web page. NIH's
Award Trends Web site now lists current and
historical data on NIH awards to states,
congressional districts, universities and other
institutions. That information is now available
through an interactive interface and through
the
NIH Research Results for the Public Web page. The new state trends funding site layout
provides the opportunity to access award
data interactively. Site visitors can click either
a specific state on the map, or the hyperlink
listed below the map to see more detail, including
dollars awarded to each congressional district and
to organizations in the selected state.
 Back
to top Back
to top |
|
NIH Encourages Data Sharing:
Multiple Resources Available |
|
 NIH
maintains a long-standing policy on sharing
results and accomplishments of NIH-funded research
with the public. As an extension of this policy,
the Office of Extramural Research (OER) introduced the
Final NIH Statement on Sharing Research Data.
Data sharing helps the community at large as it
improves access to existing resources, reduces
duplication of effort and allows the NIH to focus
on its primary goals. Today, particularly with
budget constraints, data sharing has become more
important as the NIH continues to fund
groundbreaking research. NIH
maintains a long-standing policy on sharing
results and accomplishments of NIH-funded research
with the public. As an extension of this policy,
the Office of Extramural Research (OER) introduced the
Final NIH Statement on Sharing Research Data.
Data sharing helps the community at large as it
improves access to existing resources, reduces
duplication of effort and allows the NIH to focus
on its primary goals. Today, particularly with
budget constraints, data sharing has become more
important as the NIH continues to fund
groundbreaking research.
Several resources are available on
NIH Web pages on how to develop and implement data-sharing
plans, including the
NIH Data Sharing Policy and Implementation
Guidance Web page, the
Data Sharing Policy Web page, a
Frequently Asked Questions page and the
Other Data Sharing Document and Resources page.
For issues not found in these
resources, assistance with addressing data-policy issues or for help in developing
data-sharing strategies, contact
J.P. Kim (301-435-0679),
Division of Extramural Inventions & Technology
Resources (DEITR) within the Office of Policy
for Extramural Research Administration (OPERA),
OER.
 Back
to top Back
to top |
|
PEER Reviewer informatioN: |
|
Pilot Study in Progress—Air Travel Costs for NIH Peer Reviewers under Evaluation
|
|
 Increasing
costs in airfares and fuel prices have a direct
impact on the budget of the NIH Scientific Review and Evaluation Award program, under which NIH Scientific Review Meetings are held and travel arrangements are made for NIH Peer Reviewers. As a general policy, NIH has typically provided fully refundable air travel tickets to Scientific Reviewers. As a proactive alternative, NIH Institutes and Centers including CSR, NIDCD, NIDA, NIEHS, NIA, NIDDK, NICHD, NINDS, and NCRR
are participating in a pilot study whereby World Travel Services (one of NIH’s travel service agencies) will purchase non-refundable (restricted coach) fares for Scientific Reviewers. The pilot study will compare costs between restricted fares and unrestricted fares. Participation in the pilot started on May 28, 2006, and will continue through December 31, 2006. The data analysis of the study will be assessed by the NIH Scientific Review and Evaluation Governance Committee. Should cost savings be realized, the Committee will consider recommending an NIH non-refundable airfare policy for Scientific Reviewers. Increasing
costs in airfares and fuel prices have a direct
impact on the budget of the NIH Scientific Review and Evaluation Award program, under which NIH Scientific Review Meetings are held and travel arrangements are made for NIH Peer Reviewers. As a general policy, NIH has typically provided fully refundable air travel tickets to Scientific Reviewers. As a proactive alternative, NIH Institutes and Centers including CSR, NIDCD, NIDA, NIEHS, NIA, NIDDK, NICHD, NINDS, and NCRR
are participating in a pilot study whereby World Travel Services (one of NIH’s travel service agencies) will purchase non-refundable (restricted coach) fares for Scientific Reviewers. The pilot study will compare costs between restricted fares and unrestricted fares. Participation in the pilot started on May 28, 2006, and will continue through December 31, 2006. The data analysis of the study will be assessed by the NIH Scientific Review and Evaluation Governance Committee. Should cost savings be realized, the Committee will consider recommending an NIH non-refundable airfare policy for Scientific Reviewers.
For additional information, visit the Center for
Scientific Review's
Peer Review Notes.
 Back
to top Back
to top
NIH Peer Reviewers Required to Renew CCR Registration

The
Central Contractor Registration
(CCR) is the primary payment database for the
U.S. Federal Government. CCR validates the payee
information and electronically shares the secure
and encrypted data with the federal agencies’
finance offices to facilitate paperless payments
through electronic funds transfer (EFT). NIH
peer reviewers are required to complete a
one-time registration to provide basic
information relevant to financial transactions
and keep this information current with a once
yearly renewal. Peer reviewers will be asked to
renew their registration to maintain an active
status.
NIH peer reviewers who registered last summer in preparation for
October/November 2006 review meetings will be receiving an e-mail from CCR
(subject line: CCR 30 Day Renewal Notice) with an expiration notice. Please
promptly complete the renewal with the instructions provided. If you have any
questions, please contact Mr. David
Faustin by email or 301-435-1129 by telephone, of the NIH Scientific Review Evaluation Award
(SREA) Service Center.
 Back
to top Back
to top |
|
OLAW
NEWS: |
|
OLAW
Publishes Brochure for
Investigators |
|
 The Office of Laboratory Animal Welfare's (OLAW) new brochure communicates to
investigators their responsibilities under PHS Grants Policy and PHS Policy on
Humane Care and Use of Laboratory Animals. What Investigators Need to Know About the Use of Animals
is a succinct resource for investigators who need
to know the main expectations and requirements
when animals are involved in PHS supported
research. The Office of Laboratory Animal Welfare's (OLAW) new brochure communicates to
investigators their responsibilities under PHS Grants Policy and PHS Policy on
Humane Care and Use of Laboratory Animals. What Investigators Need to Know About the Use of Animals
is a succinct resource for investigators who need
to know the main expectations and requirements
when animals are involved in PHS supported
research.
 Back
to top Back
to top
The NIH seeks applications for the position of
Director, OLAW, located in the Office of
Extramural Research (OER). The OLAW Director
provides executive leadership and direction to
OLAW, which is responsible for developing and
coordinating appropriate PHS regulations,
policies, and procedures on the humane care and
use of laboratory animals. The OLAW Director
reports directly to the NIH Deputy Director for
Extramural Research.
For information concerning the duties and
responsibilities of this position, salary and
benefits available, required qualifications and
application procedures, interested
candidates should visit the
OER
Senior Level Vacancies Web site.
 Back
to top Back
to top |
|
|
|
 
|
|
  |
|
|
The
NIH Extramural Nexus is a bimonthly update
from the NIH Office of Extramural Research. Articles,
comments, questions and suggestions may be addressed to
the Editor.
The
NIH Extramural Nexus reserves the right to
select and edit items submitted for inclusion.
To subscribe to the NIH Extramural Nexus, send a
plain text email to Listserv@list.nih.gov
including only the words Subscribe EXTRAMURALNEXUS
in the body of the message. To unsubscribe, follow the
same procedure, using the words Unsubscribe
EXTRAMURALNEXUS in the message body.
|
|
|
NIH
Extramural Nexus Web site and archives |
|
|
