
|

Notice to Readers: Revised Technical Instructions for
Tuberculosis Screening and Treatment for Panel Physicians
CDC's Division of Global Migration and Quarantine (DGMQ) has released the
2007 Technical Instructions for Tuberculosis Screening and Treatment for Panel
Physicians. These revised technical instructions and a list of the
populations whose applicants for U.S. immigration are being screened in accordance with them are available at
http://www.cdc.gov/ncidod/dq/panel_2007.htm.
CDC has U.S. regulatory authority over overseas medical examinations for immigrants, refugees, and asylees immigrating
to the United States.* Panel physicians, who are appointed
by the U.S. Department of State, perform overseas
medical examination of applicants for U.S. immigration in accordance with technical instructions provided by DGMQ.
The previous technical instructions for tuberculosis screening have been in effect since 1991. Under the
1991 instructions, applicants aged >15 years are required to have a chest radiograph and provide three sputum smears
for acid-fast microscopy if the chest radiograph is suggestive of active tuberculosis; however, no cultures (or
drug-susceptibility testing) are required. Applicants who are
sputum smear-positive must undergo treatment until they
are smear-negative before traveling to the United States, but the 1991 requirements do not specify the therapy they
should receive (1). In addition, the screening algorithms used for the 1991 instructions are insensitive and miss
smear-negative, culture-positive cases (2); the algorithms also are inadequate to prevent importation of multidrug-resistant
tuberculosis into the United States (3).
To address these shortcomings, CDC has updated the tuberculosis technical instructions, and several
important changes have been introduced. Applicants with chest
radiographs suggestive of tuberculosis now are required to
submit
three sputum specimens for both sputum smears for acid-fast microscopy and mycobacterial culture.
Drug-susceptibility testing is required to be performed on positive cultures. Before immigrating to the United States,
applicants in whom tuberculosis disease has been diagnosed must complete treatment, which must be administered according to
American Thoracic Society/CDC/Infectious Diseases Society of America guidelines and under a directly observed therapy
(DOT) program. Guidance has been added for evaluation of contacts of tuberculosis patients.
To reduce the risk of immigrants becoming infected with tuberculosis or activating latent disease, the period for which the examination is valid for travel
has been reduced to 6 months for applicants with a normal evaluation and 3 months for applicants with a Class
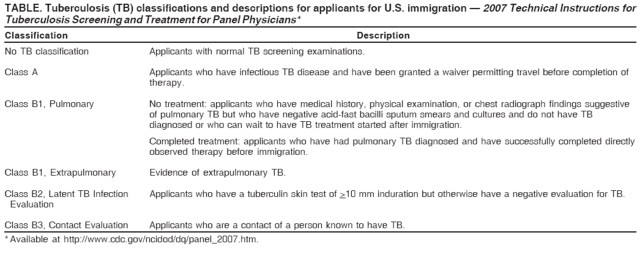
B1 classification (Table). The tuberculosis classification system also has been modified to better reflect the tuberculosis status
of the applicant and to help ensure follow-up upon arrival in the United States.
CDC is working with the U.S. Department of State, panel physicians, the International Organization for
Migration, and other organizations to implement these changes. The technical instructions are being implemented first in
priority countries, as determined by immigration patterns and tuberculosis burden. By the end of 2007, applicants for
U.S. immigration screened according to the new technical instructions included all applicants from Mexico, the
Philippines, Nepal, and Thailand. CDC will notify state and local health departments when panel physicians in a country
begin implementing this revised algorithm.
References
- CDC. 1991 technical instructions for panel physicians. Atlanta, GA: US Department of Health and Human Services, CDC; 1991. Available at
http://www.cdc.gov/ncidod/dq/panel_1991.htm.
- Maloney SA, Fielding KL, Laserson KF, et al. Assessing the performance of overseas tuberculosis screening programs: a study among
U.S.-bound immigrants in Vietnam. Arch Intern Med 2006;166:234--40.
- CDC. Multidrug-resistant tuberculosis in Hmong refugees resettling from Thailand to the United States, 2004--2005. MMWR 2005;30:741--4.
* Medical examination of aliens. 42 CFR, Part 34.
Table
Return to top.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication. |
Disclaimer
All MMWR HTML versions of articles are electronic conversions from ASCII text
into HTML. This conversion may have resulted in character translation or format errors in the HTML version.
Users should not rely on this HTML document, but are referred to the electronic PDF version and/or
the original MMWR paper copy for the official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents,
U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800.
Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
Date last reviewed: 3/19/2008
|

|
