 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Effect of New Susceptibility Breakpoints on Reporting of Resistance in Streptococcus pneumoniae --- United States, 2003In January 2003, the National Committee for Clinical Laboratory Standards (NCCLS) finalized new breakpoints for defining the susceptibility of Streptococcus pneumoniae isolates to cefotaxime and ceftriaxone (1). The former breakpoints were based on attainable concentrations of these antibiotics in cerebrospinal fluid (CSF) and the level at which it was thought that meningitis treatment failed because of elevated minimum inhibitory concentrations (MICs). The new breakpoints differ for S. pneumoniae isolates causing meningitis and those causing nonmeningeal clinical syndromes. To assess the effect of these new criteria on reporting of nonsusceptible S. pneumoniae isolates, CDC analyzed cefotaxime MIC data from the Active Bacterial Core Surveillance (ABCs) of the Emerging Infections Program (EIP) Network during 1998--2001. This report summarizes the results of that analysis, which indicated that after the new criteria were applied, the number of isolates defined as nonsusceptible to cefotaxime decreased 52.1%--61.2% for each year. Laboratory reports for clinicians should include interpretations using the new breakpoints for meningitis and nonmeningeal syndromes for all non-CSF isolates. During 1998--2001, ABCs/EIP surveillance areas from eight states (California, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee) conducted surveillance for invasive pneumococcal disease. Surveillance populations ranged from approximately 17.4 million in 1998 to 18.6 million in 2001 (2). A case of invasive pneumococcal disease was defined as isolation of S. pneumoniae from a normally sterile site in a resident of a surveillance area. Isolates were tested for susceptibility at reference laboratories by using NCCLS methods (1). Isolates were considered to be nonsusceptible to an antibiotic if they met intermediate or resistant criteria by MIC testing. Under the former criteria, susceptible, intermediate, and resistant MIC breakpoints for cefotaxime and ceftriaxone were <0.5, 1, and >2 µg/mL, respectively, for all pneumococci. Under the new criteria, isolates from CSF or other body sites where meningitis is suspected maintain the old breakpoints, but isolates causing nonmeningeal syndromes have breakpoints of <1, 2, and >4 µg/mL, respectively. During 1998--2001, the number of S. pneumoniae isolates collected annually ranged from 3,128 to 3,961 (Table). Approximately 95.6% of isolates collected caused nonmeningeal clinical syndromes such as pneumonia with bacteremia. The percentage of isolates causing meningitis ranged from 4.4% in 1998 to 5.5% in 2000. The percentage of isolates causing nonmeningeal syndromes that were nonsusceptible to penicillin ranged from 24.3% in 1998 to 26.5% in 2000. Penicillin nonsusceptibility was consistently higher among isolates causing meningitis (Table). The susceptibility breakpoints for penicillin remain unchanged and are the same for isolates causing both meningitis and nonmeningeal syndromes. Under the former breakpoints, the percentage of isolates causing nonmeningeal syndromes that were nonsusceptible to cefotaxime ranged from 13.8% in 1998 to 16.7% in 2000 (Table). Cefotaxime nonsusceptibility was consistently higher among isolates causing meningitis. When the new breakpoints were applied, the percentage of isolates causing invasive nonmeningeal syndromes defined as cefotaxime nonsusceptible decreased to 5.6%--7.4%; the percentage of isolates causing meningitis defined as nonsusceptible remained unchanged. Cefotaxime nonsusceptibility among all isolates was 6.4%--8.1%, representing a decrease of 52.1%--61.2% in cefotaxime nonsusceptibility annually (Table). Reported by: P Daily, MPH, California Emerging Infections Program, San Francisco, California. M Farley, MD, Emory Univ School of Medicine, Atlanta, Georgia. JH Jorgensen, PhD, Univ of Texas Health Science Center, San Antonio, Texas. N Barrett, MS, Connecticut Dept of Public Health. L Thomson Sanza, Maryland Dept of Health and Mental Hygiene. A Glennen, Minnesota Dept of Health. N Dumas, New York State Dept of Health. J Hatch, Oregon Dept of Human Svcs. A Craig, MD, Tennessee Dept of Health. RR Facklam, PhD, CG Whitney, MD, Div of Bacterial and Mycotic Diseases and Active Bacterial Core Surveillance of the Emerging Infections Program Network, National Center for Infectious Diseases; CM Greene, MD, EIS Officer, CDC. Editorial Note:When the new breakpoints were applied to previously collected ABCs MIC data for 1998--2001, the number of S. pneumoniae isolates defined as nonsusceptible to cefotaxime decreased 52.1%--61.2% each year. Although breakpoints remain unchanged for pneumococci from CSF or other body sites where meningitis is suspected, these isolates constitute only a small fraction (4%--5%) of all collected. Under the former criteria, S. pneumoniae infections treated with beta-lactam antibiotics to which isolates had intermediate resistance were associated with worse clinical outcomes for meningitis (3,4) but not for pneumonia (5). This difference might be related to the attainable concentration level of beta-lactam antibiotics in CSF, compared with plasma and interstitial fluid. Beta-lactam antibiotic concentrations in the lung interstitia are similar to those measured simultaneously in serum, and concentrations in CSF are lower than serum levels (6). MIC breakpoints for penicillin were not changed because susceptibility to penicillin (MIC <0.06 µg/mL) is used to predict susceptibility to other penicillins, cephalosporins, and carbapenems. Defining new penicillin susceptibility breakpoints for nonmeningeal syndromes also would require recommending specific doses for each route of penicillin administration. State and local health departments conduct surveillance for drug-resistant S. pneumoniae and rely on data generated by clinical laboratories. The change in susceptibility breakpoints will cause an artificial decline in the percentage of nonsusceptible S. pneumoniae isolates on surveillance reports. Health departments should examine laboratory data collected as part of surveillance programs to ensure that data are interpreted and aggregated correctly. Antimicrobial susceptibility testing influences clinicians' antibiotic choices (7). Current recommendations for treating penicillin-resistant pneumococcal pneumonia suggest choosing one of the following agents on the basis of susceptibility testing results: cefotaxime, ceftriaxone, selected fluoroquinolones, or, if the isolate is resistant to fluoroquinolone and cephalosporin, vancomycin (8). New clinical-syndrome--based susceptibility breakpoints for cefotaxime and ceftriaxone might lead to an increase in use of these antibiotics to treat nonmeningeal pneumococcal disease over broader-spectrum antibiotics (e.g., fluoroquinolones). S. pneumoniae strains resistant to fluoroquinolones are uncommon, but development of resistance is a concern (9). If the new NCCLS susceptibility breakpoints promote using narrower-spectrum antibiotics to treat pneumococcal disease, development of resistance to broader-spectrum antibiotics might be slowed. References
Table 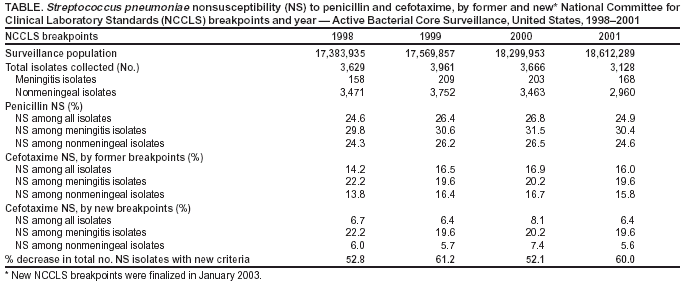 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 2/26/2004 |
|||||||||
This page last reviewed 2/26/2004
|