 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Update: HIV-2 Infection Among Blood and Plasma Donors -- United States, June 1992-June 1995Human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) both cause acquired immunodeficiency syndrome (AIDS). Following the licensure of combination HIV-1/HIV-2 screening enzyme immunoassays (EIA), the Food and Drug Administration (FDA) recommended that beginning in June 1992 all donated whole blood, blood components, and source plasma be screened for antibody to HIV-2 because not all persons infected with HIV-2 can be detected by HIV-1 testing (1,2). This report describes the first two cases of HIV-2 infection detected among potential blood donors since the implementation of recommended HIV-2 screening and summarizes national data about persons known to be infected with HIV-2 during December 1987-June 1995. * Donor 1 In June 1994, a blood donation was discarded after it tested positive by combination HIV-1/HIV-2 EIA and indeterminate by HIV-1 Western blot assay (WB). The donor was notified about the test results and consented to an interview and repeat testing. Testing at CDC indicated the specimen was positive by HIV-1 EIA, HIV-1 WB, HIV-2 EIA, and HIV-2 WB for research use only (RUO). Results of RUO synthetic peptide tests indicated cross-reactivity to HIV-1 and were interpreted as HIV-2 infection. The donor was born and resided in the United States. She previously had not donated blood or plasma. She reported no symptoms related to HIV infection and denied injecting-drug use, receipt of transfusions, and travel outside the United States. Since 1982, she had had four male sex partners; all were born in the United States. The HIV status of her partners is unknown, and she was unaware of any HIV-infection risks among them. She has no children. She received HIV counseling -- including instructions to refrain from donating blood, blood components, and tissues or organs -- and referral to a health-care provider. Donor 2 In November 1994, a plasma donation was destroyed after the serum tested positive by combination HIV-1/HIV-2 EIA and RUO HIV-2 WB. Attempts by the plasma center to notify the donor were unsuccessful. However, the donor independently sought HIV testing 2 weeks later at a counseling and testing site (CTS). The CTS laboratory results were HIV-1 EIA positive with an atypical HIV-1 WB indeterminate band pattern suggestive of HIV-2 infection. Subsequent testing at CDC indicated the specimen was HIV-1 EIA positive, HIV-1 WB indeterminate, HIV-2 EIA positive, and HIV-2 WB positive. RUO synthetic peptide EIA and dot blots were also positive for HIV-2. These results were interpreted as confirmed HIV-2 infection. During the follow-up interview, the male donor reported no symptoms of HIV infection. He had not previously donated blood or plasma. He was born in France and had lived in several countries in western Africa during 1979-1985 before moving to the United States. While in western Africa, he was vaccinated on two occasions with needles that were wiped with cotton and reused between patients. He also received several tattoos in Africa. Of his estimated 35 lifetime sex partners, most were African. The donor denied having had sex with men, injecting-drug use, and receipt of transfusions. He received HIV counseling -- including instructions to refrain from donating blood, blood components, and tissues or organs -- and referral to a health-care provider. U.S. Reports of HIV-2 Infection As of June 30, 1995, a total of 62 persons in the United States were reported with HIV-2 infection Figure_1. Of 58 persons for whom sex data were available, 38 (66%) were male. At least 11 of the 62 persons had an AIDS-defining condition at the time of report, and five are known to have died. Of these 62 persons, 42 (68%) were born in western Africa and two in Europe; for nine, the region of origin was unknown although four had malaria antibody profiles consistent with previous residence in western Africa. Of the nine persons with HIV-2 infection born in the United States, six were adults of whom four had either traveled to or had a sex partner from western Africa, and three were infants born to mothers of unknown national origin. Reported by: MD Herr, HIV/AIDS Epidemiology; AL Hathcock, PhD, State Epidemiologist, Delaware Div of Public Health. DW Hamaker, JM Schulte, DO, D Hoehns, BE Mitchell, MPH, Bur of HIV and STD Prevention; DM Simpson, MD, State Epidemiologist, Texas Dept of Health. Local and state health depts. Office of Blood Research and Review; Div of Transfusion Transmitted Diseases, Center for Biologics Evaluation and Research, Food and Drug Administration. Div of HIV/AIDS, National Center for Infectious Diseases; Div of HIV/AIDS Prevention, National Center for Prevention Svcs, CDC. Editorial NoteEditorial Note: In the United States, HIV-2 infection among blood donors is extremely rare. Since the implementation of combination HIV-1/HIV-2 EIA screening of blood and plasma donations, an estimated 74 million donations have been tested for HIV. Including the two cases described in this report, three cases of HIV-2 infection have been detected among blood and plasma donors in the United States; the first case was detected by HIV-1 screening in 1986 (3). These findings are consistent with previous surveys of approximately 20 million U.S. blood donations during 1987-1989 in which no blood-donor specimens with HIV-2 antibody were detected (4,5). The national blood supply is protected from HIV primarily through two methods: 1) interviewing donors about risk behaviors for HIV infection and 2) laboratory screening donations for HIV (6,7). All donations detected with HIV are excluded from any clinical use, ** and donors are deferred from further donations ***. For both donors described in this report, although no HIV risk factors were identified during the interview preceding blood donation, laboratory screening of their blood and plasma donations detected HIV infection. Subsequent testing revealed HIV-2 cross-reactivity resulting in a positive HIV-1 EIA (which would have led to exclusion even in the absence of HIV-2 testing) and a positive or indeterminate HIV-1 WB. HIV-1 is distributed worldwide and is prevalent in the United States; however, HIV-2 is endemic in western Africa with limited distribution to other regions of the world. Of the 62 persons reported with HIV-2 infection in the United States, at least 48 (77%) were born in, had traveled to, and/or had a sex partner from western Africa. In addition to detection of HIV-2 cases through blood and plasma donor screening, epidemiologic data about HIV-2 cases are collected through the CDC-supported national HIV/AIDS surveillance system and serosurveys (8,9). Because not all persons who are infected with HIV-2 donate blood or are otherwise tested for HIV-2, the number of persons reported with HIV-2 infection probably is underestimated. Nonetheless, the data from these sources indicate that HIV-2 is uncommon in the United States. Blood centers detecting a repeatedly reactive specimen by combination HIV-1/HIV-2 EIA should follow the recommended CDC/FDA testing algorithm (1). Specimens suspected of being HIV-2 positive may be referred to state health department laboratories or to CDC for confirmatory HIV-2 testing. Cases of HIV-2 infection should be reported to state and local health departments as allowed by law and/or regulation. Periodic updates about the number of persons known to be infected with HIV-2 in the United States are available from the CDC National AIDS Clearinghouse. References
* Single copies of this report will be available until August 18, 1996, from the CDC National AIDS Clearinghouse, P.O. Box 6003, Rockville, MD 20849-6003; telephone (800) 458-5231 or (301) 217-0023. ** 21 CFR section 610.45(c). *** 21 CFR section 606.160(e). Figure_1 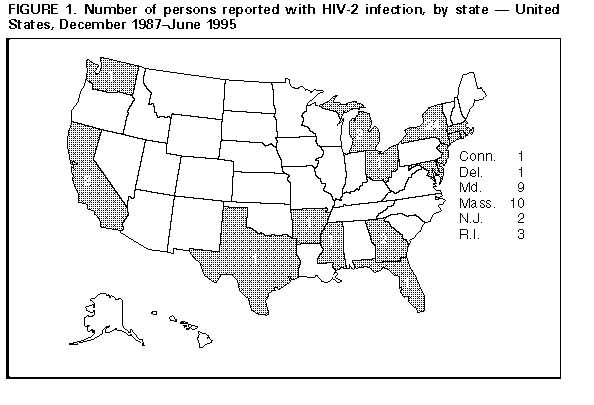 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|