 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Clostridial Endophthalmitis After Cornea Transplantation --- Florida, 2003Endophthalmitis is a severe condition caused by inflammation of the ocular cavity that often is associated with infection of the internal structures of the eye. The source of infection can include bacteria disseminated through the bloodstream and contamination of the cornea at the time of ocular surgery or trauma. Complications include rapid, irreversible vision loss that can progress quickly to panophthalmitis, requiring surgical removal of the eye (1). Clostridium perfringens, an anaerobic gram-positive bacillus found in soil and bowel flora, is an infrequent cause of endophthalmitis. Although the majority of cases are caused by penetrating injury with soil-contaminated foreign bodies, C. perfringens endophthalmitis has been reported in patients after cataract surgery (2,3). This report describes two cases of C. perfringens endophthalmitis that occurred within 24 hours after transplant of contaminated corneas. These cases demonstrate the potential for transmission of Clostridium infection from donor to recipient. Clinicians should be aware of potential infection risks associated with transplantation of corneal tissues and report any infections to the appropriate eye bank. In February 2003, two patients received corneal transplant of the right eye on the same day in the same facility. The corneas used for both patients were recovered from one donor, a woman aged 55 years who died from metastatic colon cancer. The first patient, a man aged 64 years, had severe eye pain, nausea, and vomiting within 12 hours after surgery. He had increased intraocular pressure and decreased light perception in the eye in which the cornea was transplanted. Eye examination was consistent with endophthalmitis without evidence of periorbital or orbital involvement. The patient underwent a vitrectomy and was treated with intraocular vancomycin and ceftazadime. Two days after the surgery, inflammation of the eye persisted, but no evidence of systemic illness was found. Repeat vitrectomy was performed, and clindamycin and gentamicin were injected for treatment of suspected bacillus endophthalmitis; systemic penicillin G and clindamycin were started. Cultures of fluid inside the eye yielded C. perfringens. With treatment, the patient's infection resolved; however, he continued to have minimal light perception and retinal detachment and necrosis. The second patient, a man aged 80 years, was determined on routine evaluation 1 day after surgery to have decreased visual acuity (20/400) and probable early endophthalmitis in the eye in which the cornea was transplanted. Infection progressed to severe endophthalmitis; however, he had no evidence of periorbital or orbital extension of the infection and no signs of systemic illness (Figure). Intraocular vancomycin and ceftazadime were administered. Two days after surgery, the patient's visual acuity had diminished to only light perception. The patient underwent an additional vitrectomy and was administered intraocular clindamycin and gentamicin with systemic clindamycin and penicillin G. Intraocular cultures also yielded C. perfringens. On follow-up, he recovered 20/200 vision, which was consistent with his preexisting maculopathy. Cultures of both donor corneas, collected immediately before transplantation, subsequently grew C. perfringens. Review of data from the eye bank indicated that the donor body was refrigerated within 3 hours after death; eyes were recovered approximately 8 hours after death. The corneal tissues had undergone tissue processing as recommended by the Eye Bank Association of America (EBAA) (4). The donor tissue had been maintained in a solution of gentamicin and streptomycin, and transplantation was completed within 48 hours of tissue recovery. The eye bank and the surgeon had evaluated the donor tissue by slit lamp examination and found no abnormalities. No other tissues were recovered from this donor. Both cases were reported by the eye bank to EBAA as recommended. Reported by: KG Banull, MD, JW Janelle, MD, Div of Infectious Diseases; WT Driebe, MD, Dept of Ophthalmology; LL Fauerbach, MS, Shands Hospital; L Archibald, MD, Div of Epidemiology, Univ of Florida, Gainesville. A Srinivasan, MD, D Jernigan, MD, Div of Healthcare Quality Promotion, National Center for Infectious Diseases, CDC. Editorial Note:This report describes the first reported cases of clostridial endophthalmitis associated with transplantation of contaminated corneal tissue. During 1991--2002, a total of 414,648 donor corneas were distributed for keratoplasty in the United States by EBAA-member eye banks (5). Of 230 cases of culture-positive or clinically suspected microbial endophthalmitis among corneal transplant recipients reported during 1991--2002, no cases of endophthalmitis were reported to be caused by clostridia (EBAA, unpublished data, 2003). These data indicate that corneal transplantation in the United States has a very low risk for endophthalmitis. Clostridial infections after implantation of contaminated allografts were first reported in 2001 among recipients of musculoskeletal tissues from cadaveric donors (6). In that investigation, clostridia were recovered both from tissue recipients and from the donors of the tissues. Difficulties in detecting bacteria in postprocessing cultures led to release of the contaminated allografts. Cultures of the corneas collected immediately before implantation yielded C. perfringens, indicating that the tissue donor likely had disseminated C. perfringens disease. The donor's death was attributed to metastatic colon cancer; abdominal cancer is a known risk factor for C. perfringens bloodstream infection (7). Neither cornea recipient acquired systemic infection; however, both had serious complications from infection, and one experienced substantial vision loss. The findings from this investigation underscore the serious infectious complications that can occur from transplanted allografts containing clostridia. EBAA recommends that corneal tissue should be recovered by specially trained personnel using sterile technique (4). Methods used by eye banks for processing corneal grafts include treatment with antimicrobials or bactericidal washes (e.g., povidone iodine) (8); however, these methods do not inactivate spores. Corneas used for transplant are not sterilized because existing methods (e.g., irradiation) make the tissues unsuitable for transplant. Food and Drug Administration (FDA) regulations regarding corneal tissue address the medical suitability of donors and screening for infections caused by human immunodeficiency virus types 1 and 2, hepatitis C virus, and hepatitis B virus (9). Neither FDA nor EBAA provide guidance specifically for detecting or inactivating clostridial spores on corneal allograft tissues. Cultures of corneal tissue are not performed routinely by eye banks before a corneal transplant procedure. Eye banks may elect to perform presurgical (e.g., corneal-scleral rim) cultures, and positive culture reports should be reported to the receiving surgeon or recipient eye bank. Cultures may be performed either before or at the time of surgery (4). However, presurgical cultures might not reliably predict endophthalmitis complicating corneal transplantation (10). For the two cases described in this report, culture results were not available early enough in the infection to prevent disease in recipients. If a corneal culture obtained at surgery identifies a pathogen, clinicians should evaluate the patient's condition promptly and consider initiation of appropriate therapy. Metastatic colon cancer alone is not a factor that prompts deferral of a donor; however, the medical director should evaluate information about any potential donor with metastatic colon cancer to determine whether the donation should proceed. The risk for clostridial disease from corneas should be a consideration for tissue bank directors when evaluating potential donors with metastatic colon cancer. EBAA recommends that surgeons report adverse events, including cases of C. perfringens endophthalmitis, to eye banks and subsequently to EBAA within 30 days of the occurrence for review by a medical advisory board (4). State health departments, CDC, and FDA should be notified to assist with investigations. Acknowledgments This report is based in part on data provided by EJ Holland, MD, KR Wilhelmus, MD, Eye Bank Association of America, Washington, DC. References
Figure 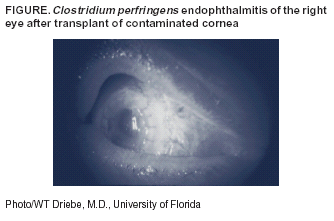 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/4/2003 |
|||||||||
This page last reviewed 12/4/2003
|