 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Progress Toward Tuberculosis Control and Determinants of Treatment Outcomes --- Kazakhstan, 2000--2002Ekaterina Bumburidi, S. Ajeilat, A. Dadu, I. Aitmagambetova, J. Ershova, R. Fagan, M.O. Favorov
Corresponding author: Michael O. Favorov, CDC, 1600 Clifton Rd, MS E-93, Atlanta, GA 30333. Telephone: 404-498-6070; Fax: 404-498-6065; E-mail: MOF0@cdc.gov. Disclosure of relationship: The contributors of this report have disclosed that they have no financial interest, relationship, affiliation, or other association with any organization that might represent a conflict of interest. In addition, this report does not contain any discussion of unlabeled use of commercial products or products for investigational use. AbstractIntroduction: In Kazakhstan, during 1995--2002, the annual notification rate per 100,000 population for new cases of tuberculosis (TB) increased from 67.1 to 165.1. Beginning in 1998, public health authorities have used the national case management strategy (DOTS) promulgated by the World Health Organization (WHO) to control TB. Intended goals of DOTS include achieving a cure rate of >85% for persons with newly detected pulmonary TB sputum-smear--positive (PTB+) cases and having PTB+ represent >65% of all PTB cases among adults. Surveillance data collected during 2000--2002 were analyzed to evaluate progress toward achieving these goals and identify factors associated with specific treatment outcomes. Methods: Surveillance data included the following nonidentifiable information on persons with newly reported cases of PTB: dates of disease onset and treatment initiation; methods of diagnosis; treatment outcomes; HIV status; and selected demographic, socioeconomic, and behavioral characteristics. Cure rates and proportions of PTB+ cases were calculated on the basis of the TB case definition and treatment outcome classification format outlined in DOTS guidelines issued by WHO. Denominator data to calculate rates were obtained from the National Census Office of Kazakhstan. Logistic regression was employed to investigate factors associated with treatment outcomes using Epi Info version 3.2. Results: During 2000--2002, a total of 65,011 new cases of PTB were detected in Kazakhstan. The average annual countrywide notification rate per 100,000 population was 146.0; provincial notification rates varied (range: 65.1--274.0). The countrywide cure rate for newly detected PTB+ was 72.2%; provincial rates varied (range: 65%--81%). Of 59,905 cases of PTB among adults during 2000--2002, a total of 26,804 (44.7%) were PTB+. Unfavorable treatment outcome of new PTB+ cases was associated with alcohol abuse, homelessness, and previous incarceration. Conclusion: The cure rate for new PTB+ cases and the proportion of cases of PTB+ among all adults with PTB were below targeted goals. This might, in part, be explained by the 1998 adoption of DOTS. Improving program indicators requires evaluation of detection efforts, laboratory diagnostic capabilities, and adherence to treatment regimens, especially in provinces in which rates are high and among persons at high risk for unfavorable treatment outcomes. IntroductionIn 1993, the World Health Organization (WHO) adopted a national case management strategy (DOTS) to reduce the increasing global burden of tuberculosis (TB), especially in developing countries (1). The five elements of the DOTS strategy are sustainable government commitment, quality assurance of sputum microscopy, standardized short-course treatment (including direct observation of therapy), regular supply of drugs, and establishment of reporting and recording systems (2,3). The goal of DOTS is to reduce TB morbidity and mortality and the chance of Mycobacterium tuberculosis developing resistance to primary treatment drugs (4). Target goals of TB control adopted in 1991 by the World Health Assembly (3) include >70% detection rate of the estimated incidence of sputum-smear--positive pulmonary TB (PTB+) and >85% cure rate for newly detected PTB+ cases. The >85% cure rate was adopted on the basis of accumulated experience in Africa and certain districts of China (5,6). Performance indicators in the DOTS program use the proportion detected of PTB+, which is the most infectious form of TB. PTB+ is associated with high mortality (7) and is the most effective form of TB to use for bacteriologic monitoring of treatment progress (8). The proportion of newly detected PTB+ cases among the total number of adults with PTB reflects the proper application of diagnostic criteria. In countries with a medium or high TB burden, when necessary laboratory resources are available and sputum smears for microscopy are taken from TB patients, PTB+ accounts for >50% of all TB cases and >65% of new PTB cases in adults (3,9). Achieving a high (i.e., >85%) cure rate for PTB+ is a critical priority for TB-control programs (3,9). Failure to achieve this rate results in continued infectiousness and possible development of multidrug-resistant (MDR) TB, which is resistant to at least isoniazid and rifampicin (10,11). During 1980--1994, the countrywide TB notification rate per 100,000 population decreased from 88.2 to 59.7. However, since 1995, notification rates have increased constantly, reaching 165.1 in 2002. TB mortality rates per 100,000 population decreased from 17.2 in 1980 to 10.6 in 1991. During 1992--1998, mortality rates increased, reaching 38.4 in 1998; however, rates decreased to 24.2 in 2002 (Kazakhstan Ministry of Health [MoH], unpublished data, 1980--2002). Increased TB mortality and morbidity have been attributed to socioeconomic changes and the deterioration of the health-care system in Kazakhstan after the country gained its independence from the former Soviet Union in 1991 (12,13). To manage the increasing burden of TB in the country, in 1998, the Kazakhstan MoH adopted and implemented a new National Tuberculosis Program (NTP), whose objectives and target goals are in accord with the DOTS strategy (14). To implement the DOTS strategy in Kazakhstan, primary health-care physicians and TB specialists received training in case-detection policy, and laboratories were equipped with binocular microscopes. During 1997--2000, the number of sputum-smear examinations increased from 661,000 to 1,170,000 (Kazakhstan MoH, unpublished data, 2001). Since 1998, a uniform TB surveillance system has operated in Kazakhstan. Beginning in 2000, all administrative territories collected surveillance data. In 2003, CDC conducted a study to evaluate NTP performance. Annual data for 2000--2002 were analyzed to describe patterns of PTB notification by person and place, assess progress toward achieving NTP target goals, and identify factors associated with unfavorable treatment outcomes. MethodsStudy DesignThe MoH surveillance database in Kazakhstan was analyzed to estimate the PTB notification rate and describe NTP performance indicators that could be used to measure progress. Selection of appropriate progress indicators was guided by WHO recommendations (15). Two performance indicators were selected: the proportion of new PTB+ cases among reported cases of PTB among adults and the proportion of persons with newly detected PTB+ cases who were cured (3). Persons in the database, registered during 2000--2002, with new PTB+ cases for which records for DOTS treatment regimen were completed by the time of the study were treated as members of a cohort that was used to study associations between treatment outcome and risk factors. The study was given institutional review board approval by the Kazakh State Medial University ethics committee. Study DefinitionsDiagnostic categories for newly reported cases of PTB (both PTB+ and sputum-smear--negative PTB) and treatment outcomes* for newly diagnosed PTB+ were defined in accordance with WHO guidelines (3). To study the associations among treatment outcome and risk factors, a new bi-level treatment outcome variable was defined on the basis of the DOTS outcome definitions: favorable (i.e., cured) and unfavorable (i.e., failed, died, or defaulted). Patients classified as having completed treatment or transferred out were excluded from the risk factor analysis because either information was lacking regarding alteration of sputum-smear status from positive to negative or they had been classified according to a new diagnostic category. Sociodemographic and behavioral variables in the surveillance database that were available for risk factor analysis included age, sex, place of residence, homelessness, employment status, employment in the medical profession, previous incarceration, and alcohol abuse. These variables were complete for >95% of records. Data were recorded by treating physicians at the time of TB diagnosis (5--10 days after hospital admission) on the basis of information provided by patients. Data SourcesBy law, TB is a reportable disease in Kazakhstan. A standard notification form is completed by the treating physician and then entered into an electronic database when a patient receives a TB diagnosis in any TB treatment facility. The national TB surveillance database contains nonidentified information on cases among civilians from all 16 administrative regions (14 provinces and two large urban areas [Astana and Almaty]); data on prisoners and military personnel are not included in this database. During 2000--2002, a total of 99,111 extrapulmonary and pulmonary cases were registered in the database; 65,011 (65.6%) were newly diagnosed PTB cases that were used for the descriptive analysis. Of these, 803 (1.2%) persons were excluded from the analysis because of lack of data on bacterioscopic investigation. Of 27,171 persons in the database with newly diagnosed PTB+, complete outcome data were available for 20,461 (75.3%) persons and were used for the risk factor analysis. Of these, 285 (1.4%) were children aged <15 years; because of these limited numbers, children were not included in the risk factor analysis. Population denominator data were obtained from the National Census Office of Kazakhstan. Statistical AnalysesData analyses were performed using Epi Info, version 3.2 (CDC, Atlanta, Georgia). Population estimates at midpoint of the 2000--2002 study period were used to calculate the average annual notification rates for new PTB by sex, age, and region. Chi-square tests were used to evaluate differences in notification rates and estimated target indicators by region. Logistic regression analysis was used to study associations between the study-defined treatment outcome variable and sociodemographic and behavioral risk factors. All variables that were significant at the two-sided a = 0.05 level in univariate analyses were included in the final multivariate logistic model; adjusted odds ratios (AORs) were used as measures of association. ResultsDescriptive StatisticsDuring 2000--2002, the countrywide average annual PTB notification rate was 146 per 100,000 population. Rates in urban and rural areas were similar (146.1 and 144.9, respectively). However, rates differed significantly (p<0.001) by region (range: 65.0 [Almaty City]--274.0 [Kzyl-Orda]). In general, the highest rates were observed in the country's western regions (Actobe, Atirau, Mangistau, and West Kazakhstan). Age-specific rate patterns were similar for men and women. The highest rates occurred among persons aged 25--34 years (men: 277; women: 241). Among persons aged >25 years, rates were consistently higher for men than for women (Figure). By region, significant differences were reported in the proportion of sputum-smear--positive cases among adults with newly reported PTB cases (range: 34.5% [Astana]--59.6% [Northern Kazakhstan]) (p<0.001) (Table 1); no region attained the goal of >65%. The cure rate for persons with new PTB+ cases varied (range: 65% [Eastern Kazakhstan]--81% [Almaty City]) (p<0.001) (Table 2). No region attained the goal of >85%. Among 99,111 persons with TB, 100 (0.1%) human immunodeficiency virus (HIV)--infected patients were identified during the study period. Risk Factor AnalysisAmong 20,176 persons aged >15 years, 547 (2.7%) patients had finished the treatment course but their sputum-smear status was unknown, and 650 (3.2%) patients were transferred out; both groups were excluded from the risk factor analysis. Of 18,979 patients included in the analysis, 4,422 (23.3%) had unfavorable treatment outcomes (i.e., died, failed, or defaulted). The proportion recorded as having an unfavorable outcome varied by age group. In univariate analyses, all studied variables were statistically significant at the 0.05 level and were included in the final multivariate logistic model. Unfavorable treatment outcomes for new PTB+ cases were associated with alcohol abuse, homelessness, previous incarceration, unemployment, being male, and urban residence. Being medical personnel was protective. AORs for unfavorable treatment outcomes increased with age (Table 3). DiscussionThe high countrywide notification rate of new PTB cases (146 per 100,000 population for the 3-year study period) underscores the importance of controlling TB in Kazakhstan. Substantial regional differences were recorded; in certain areas (e.g., Kzyl-Orda, 274 per 100,000 population), the notification rate was approximately twice the national average. As in other countries, regional differences might result from different underlying socioeconomic conditions and the quality of the performance of local TB-control programs (16,17). Further research is needed to identify the reasons for regional differences, so corrective measures can be taken as needed. The age-specific notification rate of new PTB cases was similar for children aged <15 years for both sexes. However, for persons aged >15 years, notification rates were higher among males. Similar results have been reported in other countries (18--20). The reasons for this difference in TB notification rates are not known. No evidence exists that health-seeking behaviors are substantially different in countries of the former Soviet Union and could not have accounted for male-female differences. Genetic factors or transmission dynamics might explain this difference. During 2000--2002, the proportion of PTB+ cases among the total number of new PTB cases among adults in Kazakhstan was 44.7% (program target: >65%). In certain areas, the proportion was lower (e.g., Astana-City: 34.5%; South Kazakhstan: 37.2%). The DOTS strategy was adopted recently (i.e., in 1998), and the quality of laboratory services and the application of the diagnostic criteria might not be up to the required standard, especially in certain areas. Since the DOTS strategy was implemented, the supply of primary TB drugs in TB-treatment facilities has been adequate; consequently, this factor cannot explain the low cure rate for new PTB+ cases, especially in certain regions (e.g., East Kazakhstan and Pavlodar, both 65%). Data were not available to explore reasons for the low cure rate (e.g., the prevalence of MDR-TB). The single study available indicated that high (14.2%) MDR-TB rates among new TB patients in Kazakhstan might contribute to a low cure rate in the country (21). Unemployment and urban place of residence were highly prevalent (>54%) in the study group and associated with unfavorable treatment outcomes; this could have contributed to the low cure rate. Other factors (i.e., homelessness, history of incarceration, alcohol abuse, and age >55 years) had more effect on the unfavorable treatment outcome; however, their overall effect on the cure rate might have been limited because of low prevalence. Among medical personnel, the protective odds ratio for unfavorable treatment outcome (AOR = 0.6) might be explained by their stricter adherence to treatment regimen. The high percentage of unfavorable treatment outcomes among persons aged >55 years was attributable to deaths from any cause; however, among persons in other age groups, the majority of unfavorable treatment outcomes were attributable to treatment failure. Kazakhstan is in the beginning stage of an HIV epidemic. During 2000--2002, a total of 100 (0.1%) cases of HIV were identified among 99,111 persons with TB. The contribution of HIV infection to the TB burden is believed to be insubstantial. The findings in this report are subject to at least one limitation. All study factors were measured by the treating physicians on the basis of patient self-reports. Patients might not have reported certain behaviors, particularly those regarded as socially unacceptable (e.g., alcohol abuse). As a result, the low prevalence of these behaviors among TB patients and their consequent low overall impact on the cure rate might reflect inaccurate self-reporting. Continued evaluation is needed to improve performance of the TB program in Kazakhstan. Improving program indicators requires evaluation of detection efforts, laboratory diagnostic capabilities, and adherence to treatment regimens, especially in provinces where rates are high and among persons at high risk for unfavorable treatment outcomes, so recommendations for improvement can be offered as needed. In addition, laboratory quality control and quality assurance for TB culture should be further implemented in the country to assist in assessing the impact of MDR TB on the cure rate of PTB patients. References
* Cure: person who became sputum-smear--negative in the last month of treatment and on at least one previous occasion; treatment failure: person who continued to have sputum-smear--positive status at >5 months during treatment; treatment completed: person with PTB+ who completed treatment but whose condition was not consistent with the criteria for either cure or failure; died: person who died of any cause during treatment; defaulter: person who interrupted the treatment regimen for >2 consecutive months; and transfer out: person who moved to another health-care facility and was entered in a new diagnostic category (i.e., transfer in). Table 1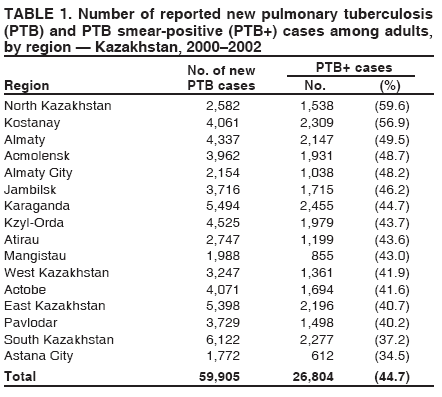 Return to top. Table 2 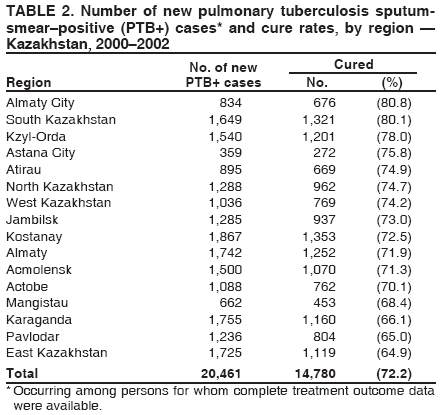 Return to top. Table 3  Return to top. Figure  Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 4/6/2006 |
|||||||||
|