
 |
|||
|
Letter to All ManufacturersSeptember 03, 2008
The purpose of this letter is to clarify the label requirements for NIOSH-approved air-purifying filtering facepiece respirators (FFR). It addresses the certification and use markings required by NIOSH to identify certified FFR classified under Subpart K, non-powered air-purifying particulate respirators, and § 84.33 (a-g) of Title 42, Code of Federal Regulations, Part 84. This letter supplements the Letter to All Manufacturers dated September 26, 1997, Subject: Clarification on Five Approval Label Issues. NIOSH is providing this clarification because current abbreviated label markings on FFR do not provide information necessary to properly identify certified FFR. Insufficient label information, lack of a testing and certification (TC) approval number marking, and the identity of the respirator manufacturer are three areas of labeling that require clarification. Significant confusion exists as to the manufacturer of FFR and as to the specific approval covering a product. FFR appear in the marketplace in small lots or are redistributed without their original bulk packaging. NIOSH receives inquiries on FFR from users who have access to none of the packaging containing the approval number and manufacturer information. Individual FFR labels will be required to have the following five distinctive markings on their exterior surface:
A sample of a generic FFR that reflects this guidance is provided as Figure 1. FFR that are private labeled are required to have the following statement on the packaging as a special “S” caution and limitation statement identified on the full label and located in the respirator user instructions: “Marketed by the private label company name and produced by the approval holder company name.” This private label related statement does not need to appear on the exterior surface of the respirator as part of the required name marking. In addition to the requirements identified above, manufacturers may wish to consider the following items in the development of their unique labels:
This policy is effective 60 days from the date of this letter for future approval requests. All currently approved FFR must meet this labeling requirement for new production within two years from the date of this policy letter. Re-labeling of existing inventory of FFR products available for purchase is not required. For additional information, please contact the NPPTL Policy and Standards Development Branch at (412) 386-5200. Jonathan V. Szalajda 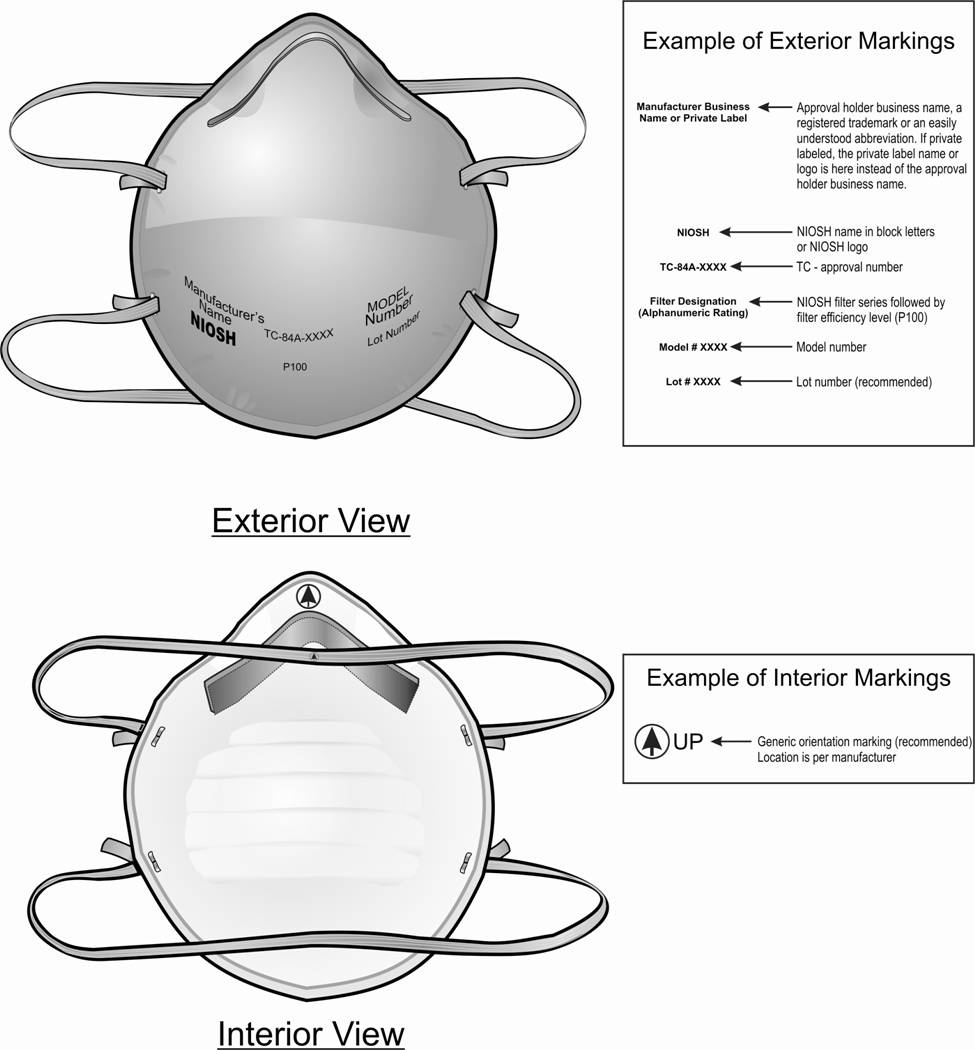
|
||||||
Figure 1. Markings. A NIOSH-approved filtering facepiece respirator depicting generic exterior and interior surface views and example marking guidance.
Printer friendly version of the: Filtering Facepiece Respirator Label
![]() PDF (212 KB, 3 page)
PDF (212 KB, 3 page)