Table
of Contents
Executive
Summary........................................................... 3
Background.......................................................................
4
I.
Family History and Chronic Disease................................ 6
II.
Analytic Validity............................................................
7
III.
Clinical Validity............................................................
14
IV.
Clinical Utility..............................................................
18
V.
Ethical, Legal, and Social Implications............................
23
VI.
Next Steps..................................................................
26
Appendix
A: Participant Roster
Appendix
B: Agenda
Appendix
C: Worksheets
Appendix
D: Selected Bibliography
Family
History as a Tool for Public Health and Preventive Medicine
Executive
Summary
On
May 1-2, 2002, the Office of Genomics and Disease Prevention (OGDP) in
the National Center for Environmental Health (NCEH), Centers for Disease
Control and Prevention, convened a workshop in Atlanta, Georgia, to
discuss the potential of family history as a public health tool for
improved disease prevention, and begin to develop a research agenda for
evaluating the feasibility, validity, and utility of this approach.
The workshop, entitled “Family History for Public Health and
Preventive Medicine: Developing a Research Agenda,” involved 36
invited participants from a variety of backgrounds and perspectives.
An
evaluation framework was used to structure the workshop presentations
and to help identify gaps in knowledge about the validity and utility of
family history information for disease prevention. The four
components of the framework were analytic validity, clinical validity,
clinical utility, and the ethical, legal and social implications of
family history screening. Presentations were also made on four
diseases as potential candidates for inclusion in a family history tool
– coronary heart disease, type 2 diabetes, asthma, and colorectal
cancer. The workshop participants discussed a number of criteria
that could be used to select the diseases to include in a public health
oriented family history tool and outlined the issues that could form the
basis of a research agenda for evaluating the validity and utility of
the tool.
By
the end of the workshop, the participants had concluded that a family
history tool for public health and preventive medicine should be: 1)
simple, easily applied, and inexpensive; 2) able to identify people at
high and moderate risk for disease; 3) useful for targeting
interventions and positively influencing healthy behaviors; and 4)
without undue cost or harm. The participants also voiced
overwhelming support for continuing both dialogue and active efforts to
fill remaining gaps in knowledge about the validity and utility of this
approach. Among the next steps outlined at the end of the workshop
were: 1) publication of articles based on the workshop presentations; 2)
formation of a working group to develop and test a family history tool;
3) identification of opportunities for using existing data to assess the
validity and utility of family history for disease prevention; and 4)
preparation of a “manifesto” that can begin to form a consensus on a
research agenda for family history.
Background
On May 1-2, 2002,
the Office of Genomics and Disease Prevention (OGDP) in the National
Center for Environmental Health (NCEH), Centers for Disease Control and
Prevention, convened a national meeting in Atlanta, Georgia, to discuss
the use of family history for improved disease prevention and
management.
Because of the
multifactorial nature of most common chronic diseases, DNA-based tests
to predict the onset of these diseases may not be available for years.
Therefore, OGDP began to think of ways to identify people who could be
at increased susceptibility for diseases that are preventable.
Family medical history seemed like a good candidate because it reflects
the consequences of inherited genetic susceptibilities, shared
environment, and common behaviors. Family history is not a new
concept; it is known to be a risk factor for most chronic diseases of
public health significance including coronary heart disease, diabetes,
several cancers, osteoporosis, and asthma. However, the collection
and interpretation of family history has rarely been applied in
preventive medicine and public health to assess disease risk and
influence early detection and prevention strategies.
A number of
methods have been proposed for quantifying the risk associated with
family history based on the number of family members affected, the
closeness of the relatives affected, and the occurrence of disease at
younger ages than would be expected. If this information
could be used to stratify the population into risk groups (i.e.,
average, moderate, and high), would people who may be at above average
risk benefit from targeted prevention and screening programs beyond what
is recommended for the population at large?
The overall
purpose of the meeting was to explore the potential of family history as
a public health tool for improved disease prevention, and begin to
develop a research agenda for evaluating the feasibility, validity, and
utility of this approach. A major component of the research would
be to evaluate simple tools for collecting family health history that
can be used in public health and preventive medicine settings.
OGDP envisions a
family history tool for public health and preventive medicine that
-
Is simple,
easily applied, and inexpensive
-
Can identify
people at high and moderate risk
-
Can be used
in combination with other risk factors
-
Is useful for
targeting interventions
-
Positively
influences healthy behaviors
-
Is amenable
to population-based use
This workshop,
entitled “Family History for Public Health and Preventive Medicine:
Developing a Research Agenda,” involved 36 invited participants from a
variety of backgrounds and perspectives (see Appendix A for participant
roster). The meeting was designed as a series of
presentations followed by intense discussion (see Appendix B for meeting
agenda). Participants were encouraged to share their thoughts and
recommendations in writing on a structured worksheet (Appendix C).
This report summarizes the meeting discussion and worksheet comments.
Specific goals of
the workshop were to
-
Generate a
list of diseases and conditions that could be included in a family
history tool, and specify criteria for selection of these diseases;
-
Describe the
specifications for a family history tool;
-
Identify gaps
in knowledge about the analytic validity, clinical validity, and
clinical utility of family history;
-
Describe the
ethical, legal and social implications;
-
Specify the
types of studies needed to fill in the knowledge;
-
Identify
existing data and studies where analysis could be done;
-
Outline new
studies and data collection that may be needed.
An evaluation
framework was used to structure the presentations and discussion.
The framework, as depicted in the wheel below, was developed by the
Foundation for Blood Research as a model process for assembling,
analyzing, and disseminating data on the safety and effectiveness of
DNA-based genetic tests and testing algorithms. The workshop
organizing committee determined that this framework could also be used
to assess the validity and utility of using family history to stratify
risk leading to improved disease prevention. The four components
of the framework are analytic validity, clinical validity, clinical
utility, and the ethical, legal, and social implications of using this
approach.
Analytic
validity addresses how accurately and reliably the tool identifies
disease among a person’s relatives. The key elements of analytic
validity are sensitivity, a measure of how well the family history tool
identifies relatives with disease, and specificity, a measure of how
well the tool identifies the relatives who do not have disease.
Clinical
validity is how well family history of disease can be used to
stratify disease risk and predict future disease in a person. The
specific elements of clinical validity include sensitivity, specificity,
and negative and positive predictive value.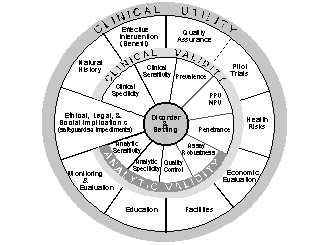
Clinical utility is an assessment of the impact and usefulness of the family history tool
for individuals, families, and society. Of particular interest is
whether the classification of individuals into risk groups would improve
the effectiveness of early diseases detection methods and interventions.
Ethical, legal
and social implications are also important because knowledge of
family history may bring unexpected stigma, psychological impact,
discrimination, informed consent requirements, and risks to privacy and
confidentiality.
The meeting began
with presentations on existing knowledge about family history as a risk
factor for four diseases – coronary heart disease, colorectal cancer,
type 2 diabetes, and asthma. Presentations followed that addressed
each of the four components of the evaluation framework – analytic
validity, clinical validity, clinical utility, and ethnical, legal and
social implications. The first section of this report
summarizes the four disease-specific presentations. The next four
sections address the components of the evaluation framework and the
questions posed during meeting discussion and in the structured
worksheet (Appendix C). The final section summarizes next steps
proposed by meeting participants. The issues and suggestions
raised here should serve as the foundation for a research and action
agenda that furthers the use of family history for public health and
disease prevention.
I. Family
History and Chronic Disease
The first four
presentations summarized the literature on family history as a risk
factor for four chronic diseases.
Coronary
heart disease
Sharon Kardia described several key studies of the family history of coronary heart
disease. Family history information that can be used to predict
disease includes: first-degree relations (father/mother), paternal
history, maternal history, paternal myocardial infarction (MI)/coronary
artery bypass grafting (CABG) before age 55 years, and maternal MI/CABG
before age 65 years. Although the results of the studies vary,
they seem to indicate that family history is a nonthreatening,
noninvasive measure of “genomic risk” or “gene-environment
combinations” for coronary heart disease and should be integrated into
public health practice. Family history is something genomic
we can do now, identifies high-risk families for primary prevention, can
be used to educate the public about genetics, can be used to assess
people’s attitudes toward the collection of genetic information, and
personalizes a family’s risk.
Colorectal
cancer
Bob Millikan discussed epidemiologic studies of family history
and cancer and their causal explanations, non-causal explanations, and
future directions. Studies consistently report a two-fold
increased risk for colorectal cancer in persons with one, or more
relatives with the disease, a finding consistent across many studies.
Causal explanations include major and minor genes, shared environmental
risk factors, or a combination of the two. Major genes syndromes,
such as HNPCC and FAP have high penetrance, but common, low penetrance
alleles such as NAT1 and NAT2 are also possible. Environmental
risk factors that family members could share include: meat intake
and cooking methods; folate, methionine, alcohol; medication use;
physical activity; and insulin resistance. Their effects may be
mediated by low penetrance alleles (gene-environment interaction).
Noncausal explanations include increased screening or access to care in
persons with affected family members, as well as recall bias. Although
what family history of colorectal cancer represents it is not yet clear,
it may be a useful proxy for a higher prevalence of at-risk genotypes
and environmental factors, especially genes and environmental factors
that interact.
Type 2
diabetes
Karen Edwards noted
that family history for type 2 diabetes has a consistent positive
association with disease and aids in risk stratification. Relative
risks ranging from 1.5 to 6, with the greater risk is associated with
earlier age of onset in relatives, number of affected relatives,
first-degree vs. second-degree relatives, and maternal effects. Issues
in assessing risk include misclassification of type 1 vs. type 2,
undiagnosed diabetes, and reduced penetrance; recall bias; confounders;
statistical issues such as differences in family size and lack of
independence; and analytic validity. Evidence is insufficient to
determine whether family history information improves early detection
and prevention of diabetes and whether it influences health promoting
behaviors.
Asthma
Wylie Burke commented on asthma, a complex disease caused by
genetic and environmental factors. A PubMed search on “asthma
& family history” and additional articles identified through
citations revealed nine relevant studies with populations greater than
750 published after 1990 addressing family history as risk for childhood
asthma. The limitations of these studies were determination
of family history by parental reporting; use of different definitions of
asthma; determination mostly by parental reporting of wheezing, current
wheeze or cough, physician-diagnosed asthma, and recent use of asthma
medications; and variation in populations by age, method of recruitment
and location. Even though they indicated that family history is
useful for identifying increased risk for asthma, the degree of risk is
uncertain. More outcome data are needed on the usefulness of
family history for primary prevention and identification of risk for
severe disease.
II. Analytic Validity
Analytic validity addresses
how accurately and reliably the tool identifies disease among a
person’s relatives. The
key elements of analytic validity are sensitivity, a measure of how well
the family history tool identifies relatives with disease, and
specificity, a measure of how well the tool identifies the relatives who
do not have disease.
Kristen Peterson provided an overview
of family history tools. Family
histories are collected by providers, insurers, employers, individuals,
genealogists, courts, social services workers researchers. Pedigrees, tables or charts, questionnaires, and narratives are
employed to gather medical and health information in families, social
and cultural traditions, behaviors and habits that impact health, and
environmental exposures. Information
is used primarily to provide care, assess risks, offer guidance, prevent
disease, aid adoptions and
social services, support genealogy, and conduct research.
The multigeneration pedigree is the gold
standard because it captures large amounts of information in a compact
standardized format that is amenable to analysis and is a dynamic
document that can be continually updated. However, the pedigree requires training and skill for maximal
effectiveness, is time consuming to collect, and is of questionable
usefulness as a screening tool.
Many other tools are available in various
formats and from different sources. They all have a common purpose:
diagnosis of a present medical condition or risk assessment to
prevent a medical condition. Using
a standard set of descriptive evaluation criteria, these tools were
characterized in terms of tool format, degree of relation, length of tool, source of tool, type of
questions, information
collected, and intended use. Several
tools were analyzed, excluding the Family Health Tree Tool described in
a later presentation. A listing of several tools can be found in
Appendix D.
Hoda Anton-Culver then discussed
issues related to validating family history tools. Family
history information about cancer is collected in clinical and research
settings and is used to infer risk of the disease in population-based,
case-control, cohort, and family-based studies. However, little information is available about the accuracy of
proband reporting. The question remains: Can
we use positive family history of cancer as a surrogate to estimate and
characterize high-risk populations? Using cancer registries, Anton-Culver
validated the reporting of family history of cancer by
cancer-affected probands in population-based and clinic-based family
registries of breast, ovarian, and colorectal cancer. The analysis found a high reliability
of reporting family history for most cancer sites among first-degree
relatives and moderate for second- and third-degree relatives. Other conclusions were that over-reporting of cancer was rare
(2.4%); race or ethnicity and sex of the proband did not influence the
accuracy of reporting; and degree of relationship to the proband, age at
diagnosis of the proband’s cancer, and source of ascertainment of
probands were statistically significant predictors of reporting.
What factors will affect analytic
validity?
Setting:
What settings are likely to yield the most valid information?
Different settings should be evaluated,
including medical offices and schools.
Take-home
questionnaires issued by physicians allow patients to learn what is
important and to query family members on their own time. Discussion can be based on completed questionnaires and the
questionnaires can be updated with physicians during annual visits.
Telephone
interviews are not effective because they rely on immediate recall.
In addition, information that can be captured by telephone is
limited (because of time, detail and mode limitations); thus telephone
interviews work best for simple information. Ideally, patients should have time before the interview to think
about the questions, look up information, and talk to relatives.
The
appropriate type of setting depends on the disease or disorder in
question. For instance, if
the condition is Mendelian, then a telephone or one-time interview may
be sufficient. However, if
the disease is multifactorial, the questionnaire should be a take- home
instrument, continuous, and validated against relatives’ information.
The setting
should encourage cooperative completion by various family members,
followed with edits by a medically knowledgeable interviewer.
Format: What formats are likely to yield the most valid information?
A combination of formats
and media is needed to reach everyone. Cultural, educational, and other personal factors must be
considered.
The appropriate format may
depend on the disease or disorder.
The format used needs to
be able to be updated and revisited periodically because family
histories change from year to year.
Web-based methods allow
easy storage, retrieval, and updating of information. Computer access is
not yet universal, however, so studying the validity of this delivery
method in lower socioeconomic and minority populations may be difficult.
An alternative is written self-administered or
interview-administered questionnaires that can be analyzed by a health
professional.
A workbook or a
computerized questionnaire can help construct a graphical family
history, as demonstrated in the SAGE-PAGE trial.
Pedigrees may be more
educational than family histories (e.g., to explain pattern of
inheritance and demonstrate disease variability).
Disease: What diseases are likely to yield the most valid information?
What criteria should be used for including specific diseases?
Validity tends to be
higher for breast, colorectal, ovarian, and brain cancer in first-degree
relatives than for cancers in other sites. Reliability for coronary
heart disease also seems to be good. On the other hand, because of the high prevalence of undiagnosed
diabetes and asthma, family histories for these diseases may be less
valid.
Reporting family history
information for diabetes may be influenced by definition of family
(biological vs. adopted); perception of condition (e.g., if taking drugs
and under control, then diabetes is not present); cultural norms
(diabetes is not considered a disease state but rather a natural part of
aging); and concerns about confidentiality (from proband and relatives).
Criteria for inclusion
need to be independent from the likely validity of family history
information.
Criteria to consider are
diseases that
-
Are common
-
Are more serious and
have a higher death rate
-
Have a defined set of
diagnostic (direct or differential) criteria
-
Are preventable, with
available interventions or simple treatments
-
Are known genetic
disorders
-
Have
an increased relative risk associated with a positive family history
and are most commonly encountered in practice or occur in the
populations
-
Have a family history
that is likely to be known, rather than undiagnosed or concealed
-
Have a good
“operational definition” of the phenotype of interest
(specificity)
Criteria should
reflect public health objectives and priorities. Taking a family history of cancer, for example, may instigate
earlier screening. regardless of the availability of genetic testing.
In addition, “screening” for some diseases may be more
acceptable than “screening” for others (e.g., family history of
suicide or depression).
Research is needed to look
at combining three or four polymorphisms or environmental factors to
produce populations of high risk.
Risk Factors: Should information about risk factors (e.g., diet,
exercise, smoking) be included in the tool?
Risk factors are helpful
for complex disease in which several genes and environmental or
lifestyle factors play a role in modifying risk. Such information allows targeted interventions to both
individuals and high-risk populations. However, it may add imprecision, unrealistically increase
questionnaire length, and therefore be less feasible in a public health
setting.
Gathering risk factor
information is especially important if it
-
Is
diagnostically relevant
-
Elicits information about tobacco and
alcohol or substance use
-
Modifies the relation with family
history
-
Relates to the research goal
Whether information
should be collected only on risk factors of the proband or also on
family members is not clear. If
the latter, the information could be linked to reflect family habits,
cultural norms and environmental factors.
Information about risk
factors should be part of the general advice offered to all patients and
should be customized for each person on the basis of the results of
family histories and exams.
Assessing diet with brief
screening tools may be difficult except for infrequent behaviors like
vegetarianism.
Other Factors:
Other factors that may
affect validity include multivitamin use and residency location of
family members.
The ultimate
criteria for a family history tool is how well it can stimulate
participation in proven interventions that prevent disease and improve
health and the impact it has on disease incidence and prevention.
The few cancer
validity studies that compare validity of data about first- and second-
degree relatives suggest lower validity for second-degree relatives.
However, inheritance patterns may be difficult to identify in
small families or for diseases affecting one sex.
A common language
that uses standard definitions and pedigree symbols is important. The symbols and definitions developed and published in 1995 are
an international standard (see Bennett et al, “Recommendations for
Standardized Pedigree Nomenclature,” Am J Hum Genet 1995;56:745-52;
and Bennett, “Practical Guide to the Genetic Family History, Chapter 4
and appendix with minimal components of family history).
If feasible, family
structure and composition should be collected once–and for all family
members–so they can be shared throughout the family without
compromising confidentiality. Then
the pedigree, with information about diseases of interest, can be
superimposed on this family structure. Details for each person could be kept private, but familial risk
stratification (“bottom line”) could be shared with all at-risk
family members. In this
way, a “skeleton” pedigree would be available for multiple uses and
users.
How can the
sensitivity and specificity of a tool be assessed?
Ideally, a gold standard
is needed, to compare information from the tool with more detailed and
valid information. This is
difficult, but one reasonable approach is to use an instrument, then
follow up with a detailed diary, in-depth interview, or record check.
The key is to determine whether the detailed interview picks up
more or different information than the form being tested.
Possible gold
standards are relatives’
self-reports, death certificates, pathology reports, and medical
records.
Sensitivity and
specificity depend not only on the disease or disorder but also on the
diagnostic criteria. Each
disease should be approached uniquely.
Not much comparative
research exists (at least for cancer) on the validity of specific tools
in assessing family history accurately, especially in a population-based
setting.
What studies
have already been done to assess analytic validity?
A small but substantial
body of literature, both in population-based and clinical settings,
deals with the assessment of sensitivity of cancer family history (less
about specificity). Validation
studies have been conducted using medical records, cancer registries,
and interviews of relatives to verify data collected from a specific
tool. However, assessment
of variables affecting reporting of cancer family history varies greatly
from study to study, and few population-based comprehensive validation
studies exist.
In addition, several
researchers have studied cardiovascular disease, substance abuse, family
violence and certain perinatal or congenital conditions (see studies by
Benson et al, and those cited by Kardia and Edwards in their
presentations).
What
studies need to be done to assess analytic validity?
Validation studies are
labor intensive and expensive when well designed, which may be why the
few existing validation studies use only one ascertainment method and do
not compare various methods.
Important studies to
pursue include
-
Assessment of negative
report validity
-
Validity of general
populations reports
-
Comprehensive,
population-based validation studies of cancer family history using
pathology or medical records as the gold standard and incorporating
all variables affecting family history report (including family
structure and tools used)
-
Analysis of the pros
and cons of approaching other family members for information
-
Determination of the
best age for taking family histories
-
Assessments of what
people know, the accuracy of their information and the means by
which the information is transmitted.
-
Validation studies of
the family history of asthma, mental illness, and diabetes
-
Ways to improve
recall, especially if the history of different diseases is assessed
at one time
-
Differences between
various methods of data collection for different populations
Study design should
ensure sufficient “power” to address the relevant questions
Do data sources exist that could address analytic
validity?
Cancer registries are good
validation sources if they have high levels of completeness and
pathology-confirmed data. Death
certificates are another good source, but their accuracy varies by
disease and is limited to confirming disease in deceased relatives.
Confirmation sources
are limited for many chronic diseases not covered in registries.
Other sources are
hospital discharge datasets; managed-care office visit data; and data
from health maintenance organizations.
What additional studies are needed?
Studies are needed to
answer the following questions
-
What is the best tool
for collecting family history compatible with use in large
populations (particularly those at moderate risk)?
-
Can the “free
market” develop an effective, user-friendly, and inexpensive
software package for documenting family history?
-
Is having an official
“family history day” worthwhile?
-
What is the validity
of reporting age at diagnosis? What are the appropriate cut-off ages to deem diagnosis or
death from various diseases premature?
-
Should family history
include all or only site-specific cancers? Should it include such conditions as benign tumors or birth
defects?
-
Which tools best
encourage recall?
-
What is the
appropriate balance between accuracy and burden of collecting family
history information?
-
What is the basis of
family history information?
-
What is the validity
of family history data collected at multiple intervals on a
population basis through an interviewer’s administered
questionnaire when the collected data are verified by registries or
another gold standard?
-
What are the core
elements of family history?
-
What is the validity
of a report of family history for primary relatives compared with
that of grandparents or other secondary relatives?
-
If only first-degree
relatives were included in a family history, how many individuals at
high risk for disease would be missed?
-
What strategies should
be adopted to motivate probands to share and discuss information
about family histories with their relatives?
III.
Clinical Validity
Clinical
validity
addresses how well family history of disease can be used to stratify
disease risk and predict future disease in a person. The specific elements of clinical validity include sensitivity,
specificity, and negative and positive predictive value.
Maren Scheuner
discussed family history collection and pedigree analysis
for chronic disease. Occasionally
single-gene disorders exist, but inheritance is more often
multifactorial with combinations of genes and environmental factors.
Characteristics associated with genetic susceptibility to
chronic disease are early onset of disease; risk for multifocal or
recurrent disease; aggressive course; resistance to conventional risk
factor modification and therapies; the same or related conditions in
affected family members; and occurrence of conditions consistent with a
known genetic syndrome. Genetic
risk can be measured in a clinic using a lab test, physical exam or
procedures, or pedigree analysis. A
review was conducted of pedigrees for coronary artery disease, stroke,
diabetes, and cancer (colon, breast, endometrial, ovarian, and
prostate). Results were
stratified by average risk (sporadic), moderate risk (familial), and
high risk (hereditary). Researchers
concluded that family history for genetic risk identification and
stratification is comprehensive,
prevalent, quantitative, qualitative, and accurate.
Joelyn Tonkin
reported on the extent to which Americans know their
family histories of asthma and heart disease. Using a nationwide, population-based survey called Healthstyles
that is conducted each year by CDC, data were analyzed to determine
the proportion of people who can report a complete family history;
review the association between incomplete family history knowledge and
demographic and health related variables; and determine whether useful
information about disease risk can be obtained from people reporting
incomplete family histories. Study
findings indicated that most people can report complete family history;
some characteristics may be related to reporting an incomplete family
history; disease status is associated with knowledge of family history;
and some family history information is better than none.
Ingrid Hall explored family history of cancer and cancer screening in
the general population. Her
research used the Cancer Control Topical Module of the 2000 National
Health Interview Survey to determine the prevalence of cancer screening
behaviors among persons with and without a first-degree family history
of breast, prostate, and colorectal cancers. She concluded that 1) persons with a family history of cancer are
more likely to be screened, regardless of test (and are also more likely
to receive screening according to recommended guidelines for mammography
and colonoscopy or flexible sigmoidoscopy); 2) although most respondents
received screening during a routine physical, a small proportion of
those with a family history sought out screening; and 3) the lack of a
screening recommendation from a physician was a common reason for not
being screened among the never screened. Although no guidelines exist for PSA testing, persons with a
family history of prostate cancer were more likely to be screened within
the past year.
What
factors will affect the clinical validity of the tool?
Relatives:
Should more than first-degree relatives be included in the tool?
Studies of gene
characterization will lead ultimately to the assessment of clinical
validity. For some of
these, full pedigree information may be necessary.
The more family
history knowledge, the better. Information
should be gathered on parents, children, siblings, aunts and uncles,
first cousins, and grandparents from both paternal and maternal sides.
The inclusion of
extended family members depends on the value of added information
relative to the effort needed to gather it. It also depends on the age of the proband and on what is best for
a valid prediction.
Disease: What diseases are likely to yield the most valid information?
Diseases likely to yield
more valid information are
-
Diseases people are
concerned about–cancer (especially breast and colon) and heart
disease
-
Serious diseases and
those that shorten lifespan
-
Diseases that produce
physically evident symptoms
-
Diseases involving
surgery
-
Common
diseases–diabetes, coronary artery disease, cardiovascular
disease, cancers, Alzheimers disease, and asthma
-
Diseases associated
with higher mortality e.g., ovarian cancer, cardiovascular disease
associated with a salient event (e.g., myocardial infarction or
stroke)
-
Diseases with better
case definitions and well-established diagnostic criteria
-
Diseases for which
interventions exist but are not yet applied population-wide
Whether the same
criteria are valid for a variety of adult diseases on the basis of
number of affected relatives, degree of relationship, side of family
affected, and age at onset is not clear.
Algorithms developed
to classify families for risk assessment purposes should be reviewed to
determine which are most useful.
Risk
stratification: What types
of classification systems or family history scores are useful for
stratifying risk?
Considerations include
-
Number of relatives
affected (first and second degree, respectively)
-
Laterality or
mulitfocality of the disease
-
Age of relatives at
diagnosis
First-degree relatives
plus grandparents may be the optimum combination for simplified data
collection and prediction.
Systems need to be
disease specific and adhere to the KISS (keep it simple, stupid)
principle. One option is to
set an update interval (e.g., 2 years or 5 years), supplemented with
regular or enhanced screening.
Definitions for
average, moderate, and high risk may differ for different diseases.
Continuous variables
are needed for research, incorporating degree of genetic relationship
and age of onset.
According to
Silberberg, et al., different schemas for producing family risk scores
are fairly equivalent.
People at average risk and
moderate risk may have difficulty comprehending and maintaining accurate
risk estimates. Clinicians
should carefully explain these estimates to their patients.
How can the sensitivity, specificity, and predictive value be assessed?
To advance the field of
systematic family history data collection, standardized data for
identifying family relationships should be adopted. Security measures and policies for sharing family data within
families and among clinicians would also be helpful. Uniform “tags” for type of relative (e.g., brother,
half-brother with same mother) should be used by all professionals.
Ways of labeling epidemiologic data by family clusters should
be explored. Also, ways of
linking existing geneologic data with health data should be developed
and archival pedigree showing family composition could then have an
“overlay” of disease information.
Comparative research (at least for cancer) is scant on the
validity of specific tools in assessing accurate family histories,
especially in a population-based setting.
Assessment requires
prospective studies, ideally through a national health system with
integrated medical records. Thorough family history data should include
number of relatives affected, age at diagnosis, and laterality. Data should be verified by other means.
Pilot studies are
needed of draft tools developed jointly with the professionals who will
use them.
How can the
attributable risk due to family history be determined?
Calculating attributable
risk requires
-
Gathering population
data on prevalence, both of different categories of familial risk
and of the disease or condition of interest
-
Measuring the
incidence of each disease in families with positive family history
for each disease
-
Subtracting risk for
disease among unexposed group (with no family history) from the risk
for disease among exposed group (with family history), given that
the two groups are comparable with regard to other risk factors
What studies
have already assessed clinical validity?
Relevant studies include
-
The Gail model, which
predicts risk for breast cancer on the basis of several known risk
factors including family history. The model does not account for family structure or size,
however, and does not include information about second-degree
relatives;
-
The Claus models for
breast cancer;
-
Colon cancer studies
among health professionals and nurses;
-
Studies correlating
family history information with actual genetic testing data.
What studies need to be done to assess clinical
validity?
Studies
are needed to
-
The
Characterize
penetrance (population) and identify effect modifiers for known
susceptibility genes
-
Validate
risk assessment models using existing case-control or cohort studies
that collected information about the appropriate risk factors.
(Accuracy of prediction varies because of misclassification
of risk factors, or failure to include all possible risk factors in
the model.)
-
Determine
the best way to stratify
-
Correlate
family history with genetics
-
Learn
more about genotype-phenotype correlation, controlling for shared
environmental factors
-
Determine
the predictive value of family history using prospective,
population-based studies
-
Evaluate
the feasibility of systematic family history collection in
conjunction with epidemiologic studies and random clinical trials of
prevention and treatment (to identify the differential effect on
those at higher familial risk)
-
Determine
the importance of clustering of risk factors by family history and
the age at diagnosis
-
Recommend
the interval at which family histories should be updated
-
Review
methods for examining the best threshold of information collection
in the context of clinical validity
-
Determine
the maximum number of diseases and environmental and behavioral
factors that can be included in a general family history tool before
responses and interest wane and the minimum number without
sacrificing important conditions with a strong genetic component
-
Assess
the value of having one risk figure that integrates family histories
of various relatives and weights their contributions appropriately
-
Determine
whether family histories outlived their usefulness because family
size is decreasing and diseases are being diagnosed earlier
-
Examine
the sensitivity and specificity affected by contributions from
multiple family members; size of family; number of affected
individuals; format (graphic vs. text or tabular); distance of
relative (first or second generation); age structure of family; and
age of informant
-
Explore
how far back (in generations) should family histories go to be
accurate and useful
Do data sources exist that could address clinical
validity?
Some
sources are
-
Cancer
family registries
-
Cancer genetics network
-
Data
collected in the clinical settings
-
Health
maintenance organizations that have piloted computer-generated
reminders that could be adapted to prompt physicians to conduct a
family history
-
Retrospective
cohort studies where family history and health status have been
verified for relatives and household members
IV.
Clinical Utility
Clinical
utility is an assessment of the impact
and usefulness of the family history tool for individuals, families, and
society. Given a tool that
has reasonable analytic and clinical validity, would the classification
of individuals into risk groups improve the effectiveness of available
early detection methods and interventions?
Steven Hunt introduced the session on clinical utility of family history
with a presentation titled, “Population Versus High-Risk Prevention
Strategies: Do We Need to Choose?”.
According to Hunt, family history is important to assess because
it significantly and independently predicts disease incidence and often
represents the expression of multiple risk factors. In addition, most families will have a family history of at least
one disease. Those at
highest risk need greater help but also respond in the best manner. Lastly, family history achieves the goals of both a
population and high-risk approach.
Family history is an independent predictor of disease. On a practical level, family history can be the only basis of
diagnosis, although it is difficult to know which contributing factors
are accounting for disease. To determine family diagnosis, one can look for shared genes
and risk factors in affected relatives, study unshared risk factors in
unaffected siblings, and examine whether risk factors are penetrant for
disease endpoints.
Hunt discussed the Health Family Tree Study, particularly in
relation to relative risk for stroke and coronary artery disease.
The cost of identifying high-risk families is often seen as a big
drawback of the high-risk approach because it may cost many times the
intervention. Estimated at
$27 per high-risk family ($3-$5 per family) using paper versions of the
Health Family Tree Study, the cost becomes very low ($1-$3) using an
internet version.
The benefits of using a high-risk strategy for family history
are profound. Family
histories of an entire population are collected and analyzed, families
become aware of diseases in relatives as they collect the information, a
family history report will put the family history into perspective, and
medical personnel can encourage lifestyle modification and further
assessment.
National family history screening can easily be expanded from
a school-based approach for use by public health agencies, medical
personnel (e.g., clinics, physicians) and the general public. This was done in Utah with a school-based Internet family
history protocol accompanied by a school curriculum, student and teacher
manuals, training on DVD and the Internet, and collection and reporting
tools. Efforts are underway
to make this a statewide program in Utah.
At the same time, more research is needed to assess the impact on
high- and average-risk families of collecting the family history and
receiving a family history report with health recommendations based on
that report. This impact can be assessed by change in diet, exercise,
smoking; use of medical resources; and long-term health benefits.
Janet Audrain also addressed the topic of
clinical utility, specifically addressing the question of whether awareness
of family history affects behavior change.
Using family history of breast cancer as an example, she examined
a variety of studies in terms of the impact of family history on risk
counseling, risk comprehension, and screening as well as behavioral and
psychological factors that may interact with awareness to impact
screening and health behavior. She
concluded that targeting
interventions to individuals who have a family history of disease may be
an effective strategy for improving health-promoting behaviors.
However, interventions
that focus only on increasing awareness of risk or accuracy of perceived
risk may not be sufficient for large or long-term behavior change.
The
psychological and behavioral variables that may affect the processing of
risk information or participation in health-promoting behavior need to
be considered.
Does knowing
about a family history of a particular chronic disease make people
engage in behaviors that improve health?
Are public health interventions more effective if they are
targeted to high-risk groups?
The answer to this question may vary by disease.
For example, if screening for a particular disease is already
high, the utility of family history may be low (e.g., breast cancer)
unless it can be shown that knowledge of family history will motivate
those who are not getting screened to do so.
Intervention may be more cost-effective if targeted to those
at highest risk. Intervention also may be more effective if the attributable
risk is appreciable, the cost of stratifying risk is low, and proven
interventions exist.
The school-based
approach to family history can be expanded to any type of national
assessment tool for use by public health agencies, medical personnel,
and the general public.
Although the ratio
of cases prevented to population targeted will probably increase, the
total number of cases prevented in the general population may not
necessarily increase.
A standard
definition of “public health intervention” is needed.
Thorough literature
reviews are needed to assess the state of the knowledge and design a
research agenda.
Are
individuals more motivated to improve their health if they know they may
be at higher risk than the average population?
Are they more likely to adhere to screening recommendations?\
Such knowledge clearly has some impact.
A higher level of awareness and most likely an increase in
screening exist, but changes in longer-term behavior and lifestyle are
far less definitive. People
seem more willing to make low-effort changes than larger sacrifices.
Theoretically, such changes could be maximized if people were
told their absolute risks based on their family history, and how much
behavior change could reduce that risk.
Physicians should be
trained and motivated to use family history as an effective tool. Medical school curricula should be reviewed to determine what
is currently taught and what changes are needed.
A good literature review
could help to make sense of mixed evidence.
Also valuable would be data on communication and support for
healthy behaviors in families as a result of identification of familial
risk.
More research is needed for different populations and ages.
Would
individuals in the average-risk groups become complacent and less likely
to engage in healthy behaviors? Does
the public’s perception of genetic determinism influence behavior?
Although complacency is
possible, there is no hard evidence that this will occur. Depending on the health behavior, the general public may
already be complacent because most people have a known family history of
some chronic disease.
Many of the preventive
behaviors are similar for a number of these conditions; thus, family
history might be used to encourage adoption of healthful behaviors for
everyone. Family-based
approaches can then reinforce and complement population-based
approaches.
Genetic determinism may
promote fatalism, and it affects people’s ability to accept
uncertainty of incomplete penetrance, unknown significance of mutations,
and unproven management. Physicians
have a definite role to play in improving knowledge about risk and its
prevention and management.
General public health
messages about healthy behaviors should still occur, but people at risk
due to family history could receive more intensive efforts.
Is
the use of family history to stratify risk and target interventions a
cost-effective approach?
Research is needed on
appropriate follow-up care for people who screen positive for family
history and are at high risk for disease.
Interventions need to be
defined from the public health perspective.
This approach appears to
be useful, particularly when “population” screening is not accepted
or effective. A less
expensive way to obtain relevant information is difficult to imagine.
However, this may depend on the disease and the cost of
collecting information.
Perhaps interventions or
recommendations may not change, but intensity of promotion or support in
complying with these recommendations might increase for individuals at
elevated (high or moderate) risk for disease.
Modeling would help to
determine whether risk assessment and targeted screening, which may be
expensive on an individual basis, prove less costly if all close
relatives who are at risk (by virtue of the index person’s risk
status) can also be targeted for intervention without the high cost of
initial risk assessment.
What studies have already assessed clinical utility?
Past
studies have examined
-
Change
resulting from various types of information interventions
-
The
value of family history information in increasing screening or
improving healthy lifestyle behaviors
-
Attitudes
toward genetic testing and behavioral consequences (by Eleanor
Singer, a sociologist and survey researcher at the University of
Michigan)
What studies need to be done?
Research
questions include the following:
-
What
is the added value of family history? Does identification of risk
increase primary prevention efforts?
Is it cost-effective or ethical to allocate resources for
secondary prevention based on family history?
-
Do different medical specialties need
different family history tools?
-
How
do perceptions of “high risk” differ by such variables as
culture, race, ethnicity, religion, and age?
-
What
effect might family history have on participation in healthy
behaviors of people without a positive family history?
-
Do
people need to completely understand the concept of risk to take
appropriate preventive behaviors, such as screening?
-
Can
family histories affect the health of people at moderate risk for
disease?
-
How
can family histories best be embedded into physicians’ already
busy routines?
-
When
should physicians refer their patients to a genetic counselor?
What criteria and family history results would lead to such
referrals?
-
How
are family history tools being used in the clinical setting?
-
Which
theories have the most relevance to motivating people to take
positive action on the basis of their family histories?
-
What
are the outcomes and behaviors of patients told they are at average
or above average risk in terms of their adherence to screening
recommendations and adoption of a fatalistic approach?
-
To
what extent does the intensity of promoting interventions or
recommendations for people at elevated (high or moderate) risk for
disease affect compliance with those recommendations?
-
What
is the difference in lifetime risk of disease between a group with
family history information and counseling and another group without
that information?
-
What
are the health outcomes and costs of systematic and prospective
family history as part of routine clinical care?
-
Is
perceived risk causally related to screening and health promoting
behaviors?
-
How
is information about elevated risk best communicated?
-
Does
focusing on specific prevention messages detract from others (e.g.,
give people “permission” to engage in fewer other preventive
health behaviors)?
Do data sources exist that could address clinical
utility?
Potential
data sources are
-
CDC
study on colon cancer
-
Utah
data from Family Health Tree Experience of the University of Utah
and Utah Department of Health
-
States
with active breast cancer or colon cancer programs
-
Province
of Ontario and Britain, which have instituted rule-based criteria on
referral for BRCA genetic assessment (and may be collecting relevant
data)
-
Harvard
cancer risk tool, which provides an individualized message about
cancer prevention and may be the subject of effectiveness evaluation
V.
Ethical, Legal, and Social Implications
Ethical, legal and social implications
are also important because knowledge of family history may bring
unexpected stigma, psychological impact, discrimination, informed
consent requirements, and risks to privacy and confidentiality.
Wylie Burke delivered the final
presentation of the meeting on the ethical, legal and social
implications relevant to family history. Issues addressed included avoiding harm, family history versus
predictive genetic testing, duty to warn, potential for harm, anxiety
associated with knowledge of affected relatives, human subjects in
research, and confidentiality. She concluded that family history is most useful as an
independent predictor when
preventive interventions are available and when those interventions are
imperfect, expensive, inappropriate for most people, and difficult to
prioritize.
Is
stigma associated with being at above average risk for disease?
Considerable
“inappropriate” labeling exists because of public misconceptions. Appropriate messages that will protect and motivate people
need to be developed.
Stigma is influenced by
individual, societal, and cultural factors.
It also varies by disease severity and availability of
intervention.
To advance the field
of systematic family history data collection, standardized data for
identifying family relationships should be adopted.
Security measures and policies for sharing family data within
families and among clinicians would also be helpful.
All professionals should use uniform “tags” for type of
relative (e.g., brother, half-brother with same mother) and explore ways
of labeling epidemiologic data by family clusters.
Ways of linking existing geneologic data with health data should
be developed, and archival pedigrees showing family composition could
then overlay disease information.
What is the
psychological impact to the individual on being at above average risk?
On the basis of their own
knowledge of family history, individuals may already believe they are at
high risk.
The psychological
impact of being at high risk is an area ripe for research.
The range of impacts could include fatalism (unwillingness to
make changes), unnecessary anxiety, impairment of self-image,
depression, shame, and blame.
Impact varies with
the individual and family coping style and the way in which information
is presented and used.
Does
potential exist for discrimination or adverse effects on personal and
family life?
Without more quantitative
and qualitative data, the potential for discrimination or adverse
effects on personal and family life is hard to judge.
Family history may create concerns about future decisions (e.g.,
children and marriage partners). Affected
relatives may be blamed for “causing” disease in younger family
members.
What are the informed consent requirements for collecting
medical information on individuals and family members?
Requirements should include a description of
-
Rationale for why
the consent is important
-
Benefits and risks
associated with giving consent
-
Obligations of the
consent givers and collectors
-
Specific choices
for exclusion and inclusion of specific information
Affected individuals
should be approached first to seek permission to contact relatives.
Members of the
public generally believe that family members have a duty to inform each
other of medical and genetic risk factors if that knowledge could affect
the relative’s health or health care.
However, this may vary by culture and ethnicity and should be
studied further.
A distinction should
remain between research and clinical or public health practice.
Do effective
safeguards exist that should be in place to protect privacy and
confidentiality?
Safeguards should be
comparable at least to safeguards that apply to other medical records.
They may need to be more stringent because they pertain to data
collected on other people.
Few states have laws that
protect private medical information and discrimination; thus,
comprehensive legislation is needed at the federal level.
Such laws would clearly demonstrate the public’s concern to
insurers and employers.
To develop effective
safeguards, we need more knowledge about the real (not just the
theoretical) threats.
Research is needed to
develop a “reasonable person standard”–the point at which a family
member becomes a research subject and has a right to confidentiality
protections.
The NIH Certificate of
Confidentiality may be a useful model.
Encrypted electronic
transmission should be considered.
What studies
have already assessed the ethical. legal, and social implications of the
use of family history?
Studies on genetic testing
should be examined because the issues related to predictive genetic
testing are similar to family history issues.
Other recent studies include:
-
BRCA detection
follow-up (Toader, Rowley, Genetic Testing in 1999)
-
The Hereditary
Hemochromatosis and Iron Overload Study (NIH/NHLBI), which is using
an integrated ELSI component in phenotypic and genotypic screening,
medical exams and family studies
-
Studies of risk
perception and psychological responses to cancer risk assessment
-
Studies that inform
people of at-risk genotype (e.g., alcohol use, smoking) and observe
preventive behaviors.
What studies
need to be done to assess the ELSI associated with the use of family
history?
Some
important research questions follow are
-
What
are the real risks? How can they be countered or minimized?
-
What
impact can be expected from an educational message that “everyone
is at increased risk for something?”
-
How
does family history affect insurance and employment?
-
To
what extent and how does family history affect social relationships?
How is this complicated by the various types of family
structures that exist today?
Do data
sources exist that could address these issues?
The legal and insurance industries may have useful
data.
VI.
Next Steps
Even thought much
progress has been made in using family histories to further public
health aims, more work is needed. Meeting participants
overwhelmingly supported continuing both dialogue and active efforts to
fill remaining gaps in knowledge through research and practice.
Among the immediate next steps mentioned were
-
Posting the
workshop presentations on the CDC website.
-
Publishing
articles based on the presentations.
-
Preparing a
“manifesto” that can begin to form a consensus on a research
agenda for family history
-
Considering a
resolution to make Thanksgiving Day a Family History Day
Participants also
agreed to form a small subgroup to interact with relevant groups on
family history issues, remain engaged with CDC, digest the meeting
discussion, and plot next steps. CDC offered to lead the
coordination of such a group, and several participants volunteered to
serve as members. Potential tasks include
-
Designing and
publishing a family history tool using materials already developed
by many groups. The tool must reflect consensus on the core
data elements and may indeed consist of more than one instrument–a
set of tools that can be employed in various ways in different
settings by different preventive medicine providers and public
health practitioners;
-
Validating
the tool using a chosen gold standard;
-
Field testing
the tool on a population basis. The tool should be viewed as
“Version 1.0,” with all relevant disclaimers, and its
development an iterative process. To the extent possible, the
field test should involve users–both consumers and providers–and
include social and clinical measures;
-
Establishing
baseline information about current use of family history tools and
information for prevention;
-
Preparing a
research agenda in each of the four areas of the evaluation
framework. The agenda should be multidisciplinary and focus on
filling knowledge gaps that will help validate family history tools
and their use for prevention;
-
Conducting a
literature review on whether perceived risk for common chronic
diseases can affect behavior change;
-
Exploring the
concept and content of clinical guidelines to advise providers on
how best to administer and use family history tools, what to
recommend to their patients, and how to motivate and support the
adoption of healthier behaviors;
-
Incorporating
family history questions into the CDC Behavioral Risk Factor
Surveillance System (BRFSS) so that common risk factors (e.g., diet,
exercise, smoking) can be stratified by family history risk (i.e.,
average, moderate, and high).
All of this work,
and more, will help lay the foundation for clinical trials and ultimate
widespread use of family history for prevention.
Appendix
A: Participant Roster
Appendix
B: Agenda
Appendix
C: Worksheets
Appendix
D: Selected Bibliography
|


