ISSN: 1080-6059
Volume 14, Number 10–October 2008
Research
Ecological Factors Associated with West Nile Virus Transmission, Northeastern United States
Heidi E. Brown,1 James E. Childs, Maria A. Diuk-Wasser, and Durland Fish ![]()
Author affiliation: Yale University, New Haven, Connecticut, USA
Suggested citation for this article
Abstract
Since 1999, West Nile virus (WNV) disease has affected the northeastern
United States. To describe the spatial epidemiology and identify risk factors
for disease incidence, we analyzed 8 years (1999–2006) of county-based human
WNV disease surveillance data. Among the 56.6 million residents in 8
northeastern states sharing primary enzootic vectors, we found 977 cases. We
controlled for population density and potential bias from surveillance and
spatial proximity. Analyses demonstrated significant spatial spreading from
1999 through 2004 (p<0.01, r2 = 0.16). A significant trend was
apparent among increasingly urban counties; county quartiles with the least
(<38%) forest cover had 4.4-fold greater odds (95% confidence interval [CI]
1.4–13.2, p = 0.01) of having above-median disease incidence (>0.75
cases/100,000 residents) than counties with the most (>70%) forest cover.
These results quantify urbanization as a risk factor for WNV disease incidence
and are consistent with knowledge of vector species in this area.
West Nile virus (WNV) disease arrived in the United States in 1999 in New York City, yet how the disease became established and details concerning the nature of the transmission cycle in the United States remain unclear. Experience in the northeastern United States suggests an urban concentration of human WNV disease cases (1,2); however, environmental factors, such as urbanization, that underlie the patterns of transmission to humans have not been explicitly evaluated. We used human surveillance data to describe and quantify the spread of WNV cases in the northeastern United States and empirically tested the hypothesis that human WNV disease is linked to the urban environment independent of human population density.
In the northeastern United States, a mainly urban cycle of WNV transmission is supported by the role of bird and mosquito species. This enzootic cycle occurs in urban bird species; human cases occur in late summer (2–7). Culex pipiens Linnaeus is the most commonly implicated mosquito vector in the maintenance of WNV in birds (1,2,8,9). In the northeastern United States, this species feeds on birds found in urban areas, such as the American robin (Turdus migratorius), house sparrow (Passer domesticus), and European starling (Sturnus vulgaris) (2,10). The role of Cx. pipiens mosquitoes as primary WNV vector is supported by consistent isolations of WNV from mosquitoes captured in surveillance traps (8,11–14) and by associations between virus-infected mosquitoes and dead-bird reports (15).
A more contentious issue is the role of different mosquito species in transmitting, or bridging, WNV between birds and other vertebrates, including humans. Cx. pipiens mosquitoes are known to breed in the organically rich water of artificial containers frequently found in urban areas (16–18). Habitat modeling of potential WNV vectors in the northeastern United States indicates an urban focus for Cx. pipiens mosquitoes (19). However, its tendencies to mostly feed on birds make it an unlikely bridge vector, although other researchers have suggested that this species exhibits late season host switching to humans (5). Aedes vexans and Cx. salinarius mosquitoes have been implicated as bridge vectors in this region (1–3) because of their abundance and more nonspecific feeding patterns (20). Although both are present in urban areas, other land uses have been found to be more predictive of their distribution (19). These other studies do not indicate whether human incidence would be linked to the same ecological factors driving enzootic transmission.
In this study, we explicitly tested whether both enzootic and bridge transmission occur in urban areas by evaluating human WNV disease and degree of urbanization within counties. We estimated the initial spatial spread in time to first case in Queens, New York, the site of first WNV detection (21), from 1999 through 2006. We also examined the trend for increasing incidence with decreasing forest cover while attempting to control for surveillance efforts and removing the effect of spatial proximity. The methods provide an example of how surveillance data with low spatial resolution can be used to quantify risk.
Methods
The study was focused in 8 northeastern states (Connecticut, Delaware, Massachusetts, Maryland, New Jersey, New York, Pennsylvania, and Rhode Island) where the same mosquito species are likely to act as primary vectors. States to the north of the study area have had limited numbers of cases and may involve different mosquito species. States farther south and west are likely to involve different species of mosquitoes; hybridization between Cx. pipiens and Cx. quinquefaciatus is more common in southern latitudes (16).
Human Incidence Data
We used annual numbers of human WNV cases reported to the Centers for Disease Control and Prevention (CDC) from 1999 through 2006. Human case data were acquired through multiple sources but met the CDC case definition, which includes clinical disease with laboratory confirmation. Data for 1999 were extracted from the Morbidity and Mortality Weekly Report (22), and data for 2000 were downloaded from the National Atlas website (http://nationalatlas.gov; 23). Human case data for 2001 through 2006 were downloaded from the US Geological Survey maps page (http://nationalatlas.gov/printable/wnv.html; 24). To protect anonymity, human data from these sources are compiled at the county level. All other data were aggregated by county to match this resolution.
Geographic Data
County boundaries for the United States and 2000 census data were downloaded from the National Atlas website (http://nationalatlas.gov/boundaries and http://nationalatlas.gov/people), and county centroids were identified to facilitate the calculation of distances between counties. Land-use data were downloaded by state from the US Geological Survey National Land Cover Institute (http://landcover.usgs.gov/natllandcover.php; 24). Percentage of land cover class by county was extracted by using Fragstats Software (25). Land uses classified as low-intensity residential, high-intensity residential, commercial/industrial/transportation, and urban/recreational grasses were grouped into a class called urban. Land uses classified as deciduous, evergreen, and mixed forest were grouped into a class called forest. These 2 land use types were considered biologically relevant to the study question.
Statistical Analyses
To document evidence for the temporal and spatial spread of WNV disease, we generated cumulative incidence curves by state and by year and examined the distance between counties with cases. Time-to-first-case detection (in years) was used as the outcome predicted by distance to the origin, which was Queens, New York. For distance calculations, we ignored counties reporting no WNV disease cases because the first case is theoretically still to be determined. To visualize WNV disease spread, we plotted the mean incidence by year, using the spatial statistics tools of ArcGIS (26).
Distance measures were then used to adjust for the effect of spatial proximity in the regression analyses (27). Incorporating measures of spatial proximity in a regression model removes the effect of spatial structure that might otherwise result in overestimation of the strength of the association between the outcome, WNV incidence, and the explanatory environmental variables (28,29).
Logistic regression modeling was initially used to identify the relevant predictors and to quantify their relative effects by calculation of odds ratios (ORs). Number of cases per county was standardized by using the 1990 US Census population density. Cumulative WNV disease incidence data from 1999 through 2006 were dichotomized at their median to provide 2 categories of high and low risk. Predictor variables, percent urban, percent forested, county area, and per capita county income were stratified by quartiles. Logistic models were tested by using the Hosmer-Lemeshow goodness-of-fit test. The best model was selected based on the Akaike information criterion (AIC), which is a measure of fit that accounts for the number of parameters in the model. Models within 2 AIC units are considered comparable; models within 7 AIC units have less support but are still comparable; and models with differences >10 AIC units are not comparable (30). The relationship between increasing cases and decreasing percentage of forested land was tested by using generalized least-square regression in STATA (31).
A risk model of total incidence was developed by using log (count +1) transformed incidence as the response variable and the variables identified as important in the logistic regression analyses as predictors. To obtain a better fit, predictor variables were entered as continuous values for this regression. The κ statistic was used to assess agreement greater than chance between the median dichotomized original incidence and the predicted incidence, for which <0.21 is considered slight to poor and >0.61 is considered substantial to almost perfect (32).
All models were initially run using only the land-use predictors; and the Moran I test was used to assess whether closer observations were more similar than those farther apart. This finding of an association based on spatial location could indicate that proximity, rather than environmental factors, explains the distribution of disease incidence. Distance variables control for this potential spatial proximity effect and reflect the presumed biological relationships within the data.
The models were also adjusted for surveillance effort. Human disease surveillance data must be interpreted with knowledge of the biases inherent to its collection (33). County per capita income was used as a measure of potential investment in surveillance and laboratory testing, as has been used in prior studies of surveillance for animal rabies (34).
Results
The Epidemic
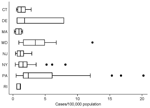 |
Figure 1. Box plot of total incidence of West Nile virus disease in humans, by county, for the 8 northeastern states in the study area... |
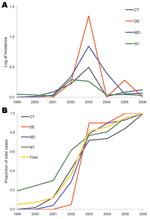 |
Figure 2. A) Epidemic curve of mean incidence (log+1 transformed) of West Nile virus disease in humans, by state, 1999–2006. The 4 states depicted are representative of the variation among the 8 states in the study area. |
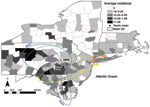 |
Figure 3. Incidence of human West Nile virus disease cases in 8 northeastern states, 1999–2006. Deviation ellipses indicate 1 SD of the geographic mean yearly incidence calculated as the incidence weighted average in space for each county... |
From 1999 through 2006, the 204 counties in the 8 states reported 977 WNV disease cases (county mean 4.8, SD 8.7, median 1, range 0–49) (Table 1). The median county incidence over the 8-year interval was 0.75 cases/100,000 residents (mean 1.77, SD 3.0, range 0–20.2/100,000). The median incidence, excluding counties with no reported cases, was 1.70/100,000 residents (mean 2.94, SD 3.45, range 0.22–20.2/100,000) (Figure 1). The highest incidence occurred in Forest County, (20.2/100,000), followed by Cameron County (16.8/100,000) and Adams County (15.3/100,000), all rural counties in central Pennsylvania with very few cases (Forest County n = 1, Cameron County n = 1, and Adams County n = 14 [13 in 2003, 1 in 2004]), and small populations, probably representing data outliers.
Associations Based on Spatial Proximity
A cursory examination of the epidemic curve of WNV cases reported from each state during the 8-year study indicated that peak incidence was broadly overlapping in all states (Figure 2, panel A). However, cumulative distribution functions of total WNV cases (Figure 2, panel B) by year indicated that New York experienced its median case earlier in the regional epidemic than did other states (Massachusetts, New Jersey, and Connecticut), which suggests a spatiotemporal spread of WNV. Because a spatial component to spread was evident, we evaluated distance between counties to assess the spatial relationship between counties and to control for the effect of spatial proximity. The spatial component alone explained 15% of the variance in time to first case when Queens, New York, was used as the origin (n = 123 counties with cases reported, p = 0.001). After 2004, no new counties reported WNV cases, and the incidence centroids of cases in 2005 and 2006 were close to one another and had shifted back toward the origin, which suggests that the disease may have reached endemicity in the region (Figure 3).
Environmental Risk Factors
Risk (high or low) for WNV cases was significantly associated (by county quartile) with measures of urbanization and with percentage of forested or urban land. Because these 2 measures were highly correlated, we used only a single measure in the final analysis (Table 2). Total county area and other demographic indices (age) were not significant predictors and are not shown.
A logistic regression of the median split for total incidence with categorical predictor variables of percentage forested area and county-based per capita income showed that percentage of forested land (χ2 = 26.13, df = 6, p<0.001) and percentage of urban land (χ2 = 5.62, df = 6, p = 0.02) were both significant predictors of incidence (Table 2). Both models provided a good fit (forested: Pearson χ2 = 7.82, df = 9, p = 0.55; urban: Pearson χ2 = 3.26, df = 8, p = 0.92). No effect of spatial proximity was found among the residuals for either model (forested: Moran I = –0.008, Z = –0.49, p = 0.31; urban: Moran I = –0.002, Z = 0.40, p = 0.34).
To adjust for surveillance bias and the spatial relationship among proximal counties, we included the variables of county-based per capita income and distance from Queens, New York, respectively (Table 2). Both forested (χ2 = 36.67, df = 11, p<0.001) and urban (χ2 = 33.55, df = 11, p<0.001) predictors were significantly associated with WNV incidence and provided a good fit (forested: Pearson χ2 = 209.27, df = 192, p = 0.19; urban: Pearson χ2 = 202.78, df = 192, p = 0.28). As before, no effect of spatial proximity was found in the residuals (forested: Moran I = –0.007, Z = –0.38, p = 0.35; urban: Moran I = 0.001, Z = 0.93, p = 0.18). Although all models were significant and fit the data, the latter model was preferred on the basis of AIC (not controlling for spatial proximity AICforested = 270.7, AICurban = 281.2; controlling for spatial proximity AICforested = 264.1, AICurban = 267.3) and included biologically relevant controls for the effect that spatial proximity might have in estimating the association between the outcome, disease incidence, and environmental variables of interest. A general, dose-dependent trend indicated increasing incidence as measures of urbanization increased (higher incidence with decreasing percentage of pixels classified as forest in each county: χ 2 = 9.47, df = 1, p<0.01; goodness of fit χ 2 = 3.50, df = 2, p = 0.17; higher incidence with increasing percentage urban land: χ 2 = 7.13, df = 1, p<0.01; goodness of fit χ 2 = 1.98, df = 2, p = 0.37).
The logistic regression model of dichotomized total incidence for the 8 years of the study, controlling for income (categorical variable by quartile) and for the effect of spatial proximity (distance variables), also showed a distinct trend of increasing incidence with percentage of forest cover; counties with <38% forest cover were 4.4× more likely (95% confidence interval 1.4–13.2, p = 0.01) to have high WNV incidence than were counties with >70% forest cover (Table 2).
Predictive Model
We used the predictors identified in the logistic regression analysis to develop a linear regression model to predict total incidence (log count + 1 transformed for a normal distribution), using the quartile percent forested land by county. Per capita income (as a continuous variable) was used to control for surveillance effort. This model explains 9.7% of the variance in the total incidence (log count + 1) (p<0.001); however, the residuals indicated an effect due to spatial proximity (Moran I = 0.0349, Z = 5.925, p<0.001). Controlling for this spatial effect and surveillance effort resulted in a better model (r2 = 0.20, p<0.001; Moran I = –0.003, Z = 0.26, p = 0.40). The κ statistic indicated good agreement (κ = 0.343, SE = 0.066, Z = 5.22, p<0.001, agreement = 67.16%) between the predicted and the observed outcomes when the binomial categorization of incidence was used and resulted in 51 county incidence entries being correctly identified as being below the median and 86 being correctly identified as being above the median. Errors were primarily in the direction of predicting the incidence above the median. When surveillance and spatial proximity were controlled for, the risk for WNV disease increased by 0.25% for every 1% decrease in forest cover. For more direct comparison with the logistic regression outcome, moving from the highest category of forest cover (>69.59%) to the lowest (<38.29%), resulted in a 6.16% increased risk for WNV disease.
Discussion
This study documents the concentration of WNV cases within urban areas of the northeastern United States and provides a quantitative estimate of the effect of varying degrees of urbanization on the risk for WNV infection at the county level. Land-use data were used to ascribe degree of urbanization as a predictor for WNV disease risk; incidence models were generated, controlling for human population density, environment-based spatial associations in the predictors, and potential biases in WNV incidence reporting resulting from the unequal resource bases among counties.
Beginning in 1999, human WNV cases were reported in counties distant from Queens, New York, the presumed origin of infection. Although the epidemic initially appeared to spread in a west/southwesterly direction in the 8-state region examined, by 2005 the initial epidemic appeared to wane, and reports of disease among newly affected counties dropped to zero. The resulting incidence maps suggest a WNV disease–endemic situation in the northeastern United States. The initial spread was not continuous along neighboring counties; rather, greater incidence was seen in urban counties after controlling for human population density, surveillance bias, and the effect of spatial proximity. The best model indicates 4× the risk for disease in the counties that fall in the lowest incidence quartile of forested land compared with the highest. The predictive nature of the data is also explored with the caveat that additional predictor variables are needed; nonetheless, it indicates increasing risk for WNV disease with decreasing forested lands.
The association between urban land use and human cases indicates that urban/suburban land use enhances environmental conditions for both enzootic and bridge transmission, at least at the county level. The spatial resolution of human surveillance data did not allow for finer evaluation of within-urban associations. Brownstein et al. linked human WNV cases to greenness indices in urban areas and found an optimal vegetation index associated with higher human cases (35). Brown et al. found an environmental separation of bridge and enzootic vectors; bridge vectors occurred in areas with vegetation that might be associated with residential areas within a city (36). Finer spatial resolution human data would allow for within-county analyses that might provide better estimations of where the cases (urban, periurban) are occurring. This would improve the predictive power of land use in the models, and the better association between land use and cases might help further elucidate which mosquito species are involved as bridge vectors.
Because of the type and resolution of the data, a sample predictive model, and not a predictive map, is provided. Nonetheless, the data and analysis provided are insightful as potentially predictive models. Additional data, such as bird abundance and perhaps also mammal abundance, are needed (37). Because of the often strict host and habitat preferences of mosquito species, mosquito surveillance data could also improve the predictive power and validity of the model. Our best predictive model explains only 20% of the variance; additional variables such as these might improve the model because the abundance of hosts and mosquito species will have a considerable effect on WNV transmission.
Despite the reluctance to use human surveillance data for models of disease transmission (33), such data can provide information about spatial associations in vector-borne disease as shown here and by others (34,38,39). This type of human surveillance modeling provides some useful insight into the distribution of human WNV cases and supports the current understanding of the transmission cycle.
To predict WNV disease requires understanding of the factors driving both enzootic transmission and bridging to humans. Different data availability and scales are involved in studying these 2 processes. We took advantage of the national coverage of the human incidence dataset to examine the spatiotemporal spread of WNV in this region and to generate a risk model based on land use, adjusted for the effect from spatial proximity. We show that human surveillance data at the county level are consistent with the urban nature of this disease system, as has been found in studies of enzootic transmission, indicating that the 2 processes occur in or near urban areas.
This work was supported by the US Department of Agriculture, Agricultural Research Service (1USDA-58-0790-5-068). H.E.B. was supported through the CDC Fellowship Training Program in Vector-Borne Disease at Yale University.
Dr Brown graduated from the Vector Ecology Laboratory at Yale University and is a researcher with the Spatial Ecology and Epidemiology Group, University of Oxford, Oxford, UK. In her research she seeks a better understanding of vector-borne disease transmission through the investigation of the relationship between vectors and the environment in which they persist.
References
- Petersen LR, Roehrig JT. West Nile virus: a reemerging global pathogen. Emerg Infect Dis. 2001;7:611–4.
- Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–74.
- Marra PP, Griffing S, Caffrey C, Kilpatrick AM, McLean R, Brand C, et al. West Nile virus and wildlife. Bioscience. 2004;54:393–402. DOI
- Roberts RS, Foppa IM. Prediction of equine risk of West Nile Virus infection based on dead bird surveillance. Vector Borne Zoonotic Dis. 2006;6:1–6. PubMed DOI
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:606–10. PubMed DOI
- Gibbs SE, Allison AB, Yabsley MJ, Mead DG, Wilcox BR, Stallknecht DE. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector Borne Zoonotic Dis. 2006;6:57–72. PubMed DOI
- Komar N, Panella NA, Burns JE, Dusza SW, Mascarenhas TM, Talbot TO. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis. 2001;7:621–5.
- Andreadis TG, Anderson JF, Vossbrinck CR. Mosquito surveillance for West Nile Virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, Culiseta melanura. Emerg Infect Dis. 2001;7:670–4.
- Turell MJ, O'Guinn M, Oliver J. Potential for New York mosquitoes to transmit West Nile Virus. Am J Trop Med Hyg. 2000;62:413–4.
- Turell MJ, Sardelis MR, Dohm DJ, O'Guinn ML. Potential North American vectors of West Nile virus. Ann N Y Acad Sci. 2001;951:317–24. PubMed
- Anderson JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, French RA, et al. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science. 1999;286:2331–3. PubMed DOI
- Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Dis. 2004;4:360–78. PubMed DOI
- Anderson JF, Andreadis TG, Main AJ, Kline DL. Prevalence of West Nile virus in tree canopy–inhabiting Culex pipiens and associated mosquitoes. Am J Trop Med Hyg. 2004;71:112–9.
- Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742–4.
- Nasci RS, Komar N, Marfin AA, Ludwig GV, Kramer LD, Daniels TJ, et al. Detection of West Nile virus–infected mosquitoes and seropositive juvenile birds in the vicinity of virus-positive dead birds. Am J Trop Med Hyg. 2002;67:492–6.
- Carpenter SJ, La Casse WJ. Mosquitoes of North America (north of Mexico). Berkeley (CA): University of California Press; 1955.
- Means RG. Mosquitoes of New York. Albany (NY): University of the State of New York, State Education Department, State Science Service, New York State Museum; 1979.
- Andreadis TG, Thomas MC, Shepard JJ. Identification guide to the mosquitoes of Connecticut. New Haven (CT): The Connecticut Agricultural Experiment Station; 2005.
- Diuk-Wasser MA, Brown HE, Andreadis TG, Fish D. Modeling the spatial distribution of mosquito vectors for West Nile virus in Connecticut, USA. Vector Borne Zoonotic Dis. 2006;6:283–95. PubMed DOI
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, et al. Host-feeding habits of Culex and other mosquitoes (Diptera : Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–85.
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile Virus disease. Emerg Infect Dis. 2005;11:1167–73.
- Centers for Disease Control and Prevention. Update: West Nile–like viral encephalitis—New York, 1999 [reprinted from MMWR; 1999:48:890–2]. JAMA. 1999;282:1714. PubMed DOI
- US Geological Survey. National atlas of the United States. 2006 May 22 [cited 2007 Jan 1]. Available from http://nationalatlas.gov
- US Geological Survey. West Nile virus: human [cited 2008 Aug 7]. Available from http://diseasemaps.usgs.gov/wnv_us_human.html
- McGarigal K. Cushman SA, Neel MC, Ene E. FRAGSTATS. In: Spatial pattern analysis program for categorical maps. Amherst (MA): University of Massachusetts; 2002.
- Environmental Systems Research Institute. I., ArcGIS. Redlands (CA): Environmental Systems Research Institute, Inc.; 2004.
- Waller LA, Gotway CA. Linking spatial exposure data and health events. In: Applied spatial statistics for public health data. New York: John Wiley and Sons; 2004.
- Legendre P. Spatial autocorrelation—trouble or new paradigm. Ecology. 1993;74:1659–73. DOI
- Lichstein JW, Simons TR, Shriner SA, Franzreb KE. Spatial autocorrelation and autoregressive models in ecology. Ecol Monogr. 2002;72:445–63.
- Burnham KP, Anderson DR. Multimodel inference—understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33:261–304. DOI
- StataCorp LP. Intercooled Stata 9.0. College Station (TX): StataCorp LP; 2005.
- Landis JR, Koch GG. Measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. PubMed DOI
- Brookmeyer R, Stroup DF. Monitoring the health of populations: statistical principles and methods for public health surveillance. Oxford (NY): Oxford University Press; 2004.
- Childs JE, Krebs JW, Real LA, Gordon ER. Animal-based national surveillance for zoonotic disease: quality, limitations, and implications of a model system for monitoring rabies. Prev Vet Med. 2007;78:246–61. PubMed DOI
- Brownstein JS, Rosen H, Purdy D, Miller JR, Merlino M, Mostashari F, et al. Spatial analysis of West Nile virus: rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne Zoonotic Dis. 2002;2:157–64. PubMed DOI
- Brown H, Duik-Wasser M, Andreadis T, Fish D. Remotely sensed vegetation indices identify mosquito clusters of West Nile virus vectors in an urban landscape in the northeastern US. Vector Borne Zoonotic Dis. 2008;8:197–206. PubMed DOI
- Kramer LD, Bernard KA. West Nile virus infection in birds and mammals. Ann N Y Acad Sci. 2001;951:84–93. PubMed
- Lucey BT, Russell CA, Smith D, Wilson ML, Long A, Waller LA, et al. Spatiotemporal analysis of epizootic raccoon rabies propagation in Connecticut, 1991–1995. Vector Borne Zoonotic Dis. 2002;2:77–86. PubMed DOI
- Waller LA, Goodwin BJ, Wilson ML, Ostfeld RS, Marshall SL, Hayes EB. Spatio-temporal patterns in county-level incidence and reporting of Lyme disease in the northeastern United States, 1990–2000. Environ Ecol Stat. 2007;14:83–100. DOI
Figures
Figure 1. Box plot of total incidence of West Nile virus
disease in humans, by county, for the 8 northeastern states in the study area...
Figure 2. A) Epidemic curve of mean incidence (log+1
transformed) of West Nile virus disease in humans, by state, 1999–2006. The 4
states depicted are representative of the variation among the 8 states in the
study area...
Figure 3. Incidence of human West Nile virus disease cases
in 8 northeastern states, 1999–2006. Deviation ellipses indicate 1 SD of the
geographic mean yearly incidence calculated as the incidence weighted average
in space for each county...
Tables
Table 1. Incidence (per 100,000 persons) of West Nile
virus disease in humans, northeastern United States, 1999–2006
Table 2. Odds ratios for median split incidence of West
Nile virus diseases in humans, for significant variables
Suggested Citation for this Article
Brown HE, Childs JE, Diuk-Wasser MA, Fish D. Ecological factors associated with West Nile virus transmission, northeastern USA. Emerg Infect Dis [serial on the Internet]. 2008 Oct [date cited]. Available from http://www.cdc.gov/EID/content/14/10/1539.htm
DOI: 10.3201/eid1410.071396
1Current affiliation: University of Oxford, Oxford, UK.
Please use the form below to submit correspondence to the authors or contact them at the following address:
Durland Fish, Yale University, LEPH 600, 60 College St, New Haven, CT 06520, USA; email: durland.fish@yale.edu
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted September 25, 2008
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435