ISSN: 1080-6059
Volume 13, Number 9–September 2007
Letter
Disseminated Bocavirus Infection after Stem Cell Transplant
Thomas Schenk,* Brigitte Strahm,* Udo Kontny,*
Markus Hufnagel,* Dieter Neumann-Haefelin,* and Valeria Falcone* ![]()
*Freiburg University Medical Center, Freiburg, Germany
Suggested citation for this article
To the Editor: Human bocavirus (HBoV) (1) is increasingly recognized as a cause of respiratory infections worldwide. Children and infants appear to be most at risk (2–7), although HBoV's role in immunocompromised patients remains unclear. We report on a child with disseminated HBoV infection after hematopoietic stem cell transplantation (HSCT). HBoV DNA was detected at high levels in nasopharyngeal aspirates (NPAs) and in blood and stool samples.
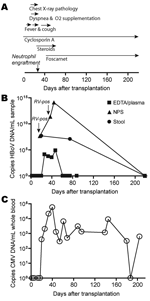 |
Appendix Figure. Timeline of clinical and virologic features posttransplantation. A) Main clinical events and therapeutic measures... |
A 4.5-year-old boy with dyskeratosis congenita was brought for treatment to our hospital due to severe persistent cytopenia. Allogenic HSCT was performed in August 2006 after conditioning with total body irradiation (200 cGy, day –8 before HSCT surgery), fludarabine (days –7 to –4), antithymocyte globulin (days –4 to –1), and cyclophosphamide (days –3 to –2). He received 7.16 × 108 nucleated bone marrow cells/kg body weight from a 9/10 human leukocyte antigen–matched unrelated donor. Graft-versus-host disease (GvHD) prophylaxis consisted of a short course of methotrexate and cyclosporin A. Neutrophil and platelet engraftment occurred on days 22 and 65 after surgery, respectively. Despite pre- and post-HSCT anti-infective prophylaxis with cotrimoxazole, colistin, acyclovir, and fluconazole, Enterobacter cloacae sepsis was diagnosed on day 2. After meropenem treatment, blood cultures remained negative. On day 12, fever reoccurred, elevated C-reactive protein values (229 mg/L) and reduced general health were noted, but no bacterial pathogen was isolated. During this period, the patient received antimicrobial drug therapy with meropenem, tobramycin, vancomycin, and amphotericin B. On day 16, his body temperature peaked to 40.6°C, and a cough and dyspnea without wheezing developed. Chest radiograph results suggested pneumonia with perihilar infiltrates. Reduced oxygen saturation (pO2 86%) was recorded transcutaneously, and oxygen supplementation (maximum 4 L/min) was started by face mask (Appendix Figure). An NPA sample investigated by multiplex PCR (results provided by W. Puppe and J. Weigl; www.pid-ari.net) was negative for adenovirus, respiratory syncytial virus, human metapneumovirus, parainfluenza viruses 1–4, influenza viruses A and B, coronavirus, reovirus, enterovirus, Clamydia pneumoniae, Mycoplasma pneumoniae, Bordetella pertussis, B. parapertussis, and Legionella pneumophila, but positive for rhinovirus RNA. Retrospectively, the same NPA sample was reanalyzed for HBoV DNA by real-time PCR (7) and showed a viral load of 4.6 × 107 copies/mL (Appendix Figure); specificity was confirmed by sequencing.
From day 19 on, the patient's general health improved and the chest radiograph results returned to normal. After neutrophil engraftment (day 22) and addition of erythromycin to the antimicrobial drug regimen, body temperature decreased and oxygen supplementation was discontinued. However, rhinitis, cough, and low-grade fever (<38.5°C) persisted until day 50 (Appendix Figure), and HBoV DNA was detected in NPAs on days 37 and 44 at 2.4 × 1011 and 1.3 × 1014 copies/mL, respectively (Appendix Figure). The NPA sample on day 37 was still rhinovirus positive.
Concurrent with the increased HBoV load in NPAs, cytomegalovirus (CMV) reactivation was first diagnosed by PCR on day 20 and peaked (58.250 copies/mL whole blood) on day 41 despite gancyclovir therapy. Switching to foscarnet led to temporary control of CMV replication (Appendix Figure). Additionally, on day 22, acute GvHD grade I with skin manifestations developed. Treatment with steroids until day 60 led to complete resolution.
HBoV infection in this patient was not restricted to the respiratory tract. Diarrheic stool samples obtained on day 21 and, after resolution of respiratory symptoms, on day 75 showed substantial HBoV DNA (2.5 × 106 and 6.0 × 105 copies/mg, respectively; Appendix Figure). Tests for rotavirus and adenovirus antigens were negative, and no bacterial pathogen was isolated. Moreover, HBoV DNA was detected at lower levels (3.7 × 103 to 7.8 × 104 copies/mL) in 4 EDTA plasma samples taken days 21–47. Subsequent plasma (days 61, 68, 75, 88, 219), NPA (day 219), and stool (day 219) samples were negative for HBoV DNA. However, the ability of HBoV to cause persistent infection, as do other members of the Parvovirinae subfamily, cannot be excluded. Future investigations are needed to address this hypothesis.
Here, we report on disseminated HBoV infection in an immunocompromised patient. Whether the clinical course in this case was more severe or prolonged than it would have been for HBoV infections in non-HSCT children remains unknown due to the lack of long-term observations in immunocompetent children. The dramatic increase of HBoV load in NPAs and viral dissemination most likely resulted from progressive impairment of cellular immunity as indicated by simultaneous CMV reactivation. Moreover, the increased viral load might have also been a consequence of steroid addition to immunosuppressive therapy to control GvHD. The contribution of HBoV to respiratory disease remains ambiguous because 2 NPA samples were also rhinovirus positive. Additional studies are required to investigate the pathogenic role of HBoV in double or multiple infections. Association of HBoV with the patient's continued diarrhea is in accordance with previous studies (8–10). Prolonged fecal shedding has important implications for isolation measures in transplantation units. More studies in immunocompromised patients are required to evaluate the spectrum of pathology caused by this emerging virus.
Acknowledgments
We are indebted to O. Haller for critically reading the manuscript and continued support and to Gudrun Woywodt for excellent technical assistance.
This work was supported by PID-ARI.net grant 01KI9910/2 from the German Federal Ministry of Education and Research.
References
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102:12891–6.
- McIntosh K. Human bocavirus: developing evidence for pathogenicity. J Infect Dis. 2006;194:1197–9.
- Ma X, Endo R, Ishiguro N, Ebihara T, Ishiko H, Ariga T, et al. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol. 2006;44:1132–4.
- Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35:99–102.
- Smuts H, Hardie D. Human bocavirus in hospitalized children, South Africa. Emerg Infect Dis. 2006;12:1457–8.
- Weissbrich B, Neske F, Schubert J, Tollmann F, Blath K, Blessing K, et al. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis. 2006;6:109.
- Schenk T, Huck B, Forster J, Berner R, Neumann-Haefelin D, Falcone V. Human bocavirus DNA detected by quantitative real-time PCR in two children hospitalized for lower respiratory tract infection. Eur J Clin Microbiol Infect Dis. 2007;26:147–9.
- Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, et al. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194:1276–82.
- Manning A, Russell V, Eastick K, Leadbetter GH, Hallam N, Templeton K, et al. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis. 2006;194:1283–90.
- Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43:283–8.
Figure
Suggested Citation for this Article
Schenk T, Strahm B, Kontny U, Hufnagel M, Neumann-Haefelin D, Falcone V. Disseminated bocavirus infection after stem cell transplant [letter]. Emerg Infect Dis [serial on the Internet]. 2007 Sep [date cited]. Available from http://www.cdc.gov/EID/content/13/9/1425.htm
Please use the form below to submit correspondence to the authors or contact them at the following address:
Valeria Falcone, Department of Virology, Freiburg University Medical Center, Hermann-Herder-Strasse 11, 79104 Freiburg, Germany; email: valeria.kapper-falcone@uniklinik-freiburg.de
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted August 29, 2007
This page last reviewed August 29, 2007
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435