|
This document updates and replaces the guidelines for specimen collection posted previously on CDC’s SARS website, to reflect the most recent information on laboratory diagnostics for SARS-CoV. The main changes are as follows:1
- Addition of stool, serum, and plasma to the list of specimens for RT-PCR testing
- Addition of information on the optimal timing of specimen collection and testing by specimen type
- Recommendation to collect multiple specimens for RT-PCR testing
1 Pending IRB approval. |
Key Messages
- Consult the state or local health department to determine the appropriateness and details of SARS testing.
- If possible, collect multiple specimens from different body sites and at different times during illness.
- A signed consent form is recommended when collecting specimens for SARS-CoV RT-PCR or antibody testing.
|
Before
collecting and shipping specimens for SARS-CoV testing, consult
with the state health department/state epidemiologist to determine
whether the patient meets the SARS
case definition. Health
department contact information is
available at: www.cste.org/members/state_and_territorial_epi.asp.
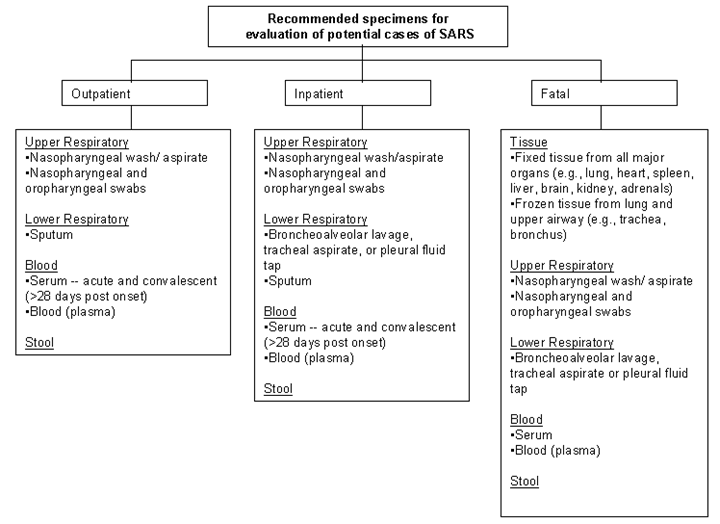
When possible, collect multiple respiratory specimens for testing. For example, collect specimens from two different sites on the same day (e.g., one nasopharyngeal swab and a stool specimen or another respiratory specimen) or from two different times during the illness. The chart on the last page specifies recommended specimen types for SARS-CoV diagnostics by stage of illness, including postmortem specimen collection.
A
signed consent form is recommended when collecting specimens
for SARS-CoV RT-PCR or antibody testing. Information on the
consent process for collection of respiratory specimens,
blood or stool for RT-PCR testing is provided at: www.cdc.gov/ncidod/sars/lab/rtpcr/index.htm.
Information on the consent process for the collection of
blood/serum for antibody testing is provided at: www.cdc.gov/ncidod/sars/lab/eia/index.htm.
RT-PCR Diagnostics
Although studies to date have not definitively determined the best specimens for SARS RT-PCR diagnostics, it is reasonable to collect:
- During the first week of illness: Nasophyaryngeal (NP) swab plus oropharygeal (OP) swab and a serum or plasma specimen
- After the first week of illness: NP swab plus OP swab and a stool specimen
Serologic Diagnostics
Serum specimens for SARS-CoV antibody testing should be collected when the diagnosis is first suspected and at later times if indicated. An antibody response is occasionally detected during the first week of illness, likely to be detected by the end of the second week of illness, and sometimes may not be detected until > 28 days after onset of symptoms. |
I. Collecting Respiratory Specimens
Eight types of respiratory specimens may be collected for viral and/or bacterial diagnostics: 1) nasopharyngeal wash/aspirates, 2) nasopharyngeal swabs, 3) oropharyngeal swabs, 4) broncheoalveolar lavage, 5) tracheal aspirate, 6) pleural fluid tap, 7) sputum; and 8) post-mortem tissue. Nasopharyngeal wash/aspirates are the specimen of choice for detection of most respiratory viruses and are the preferred specimen type for children under age 2 years.
In contrast to most respiratory pathogens for which respiratory specimens are optimally collected within 72 hours after the onset of symptoms, levels of SARS-CoV may be higher later in the course of the illness.
Before collecting specimens, review the infection control precautions in Supplement I.
A. Collecting specimens from the upper respiratory tract
- Nasopharyngeal wash/aspirate
Have the patient sit with head tilted slightly backward. Instill 1 ml-1.5 ml of nonbacteriostatic saline (pH 7.0) into one nostril. Flush a plastic catheter or tubing with 2 ml-3 ml of saline. Insert the tubing into the nostril parallel to the palate. Aspirate nasopharyngeal secretions. Repeat this procedure for the other nostril. Collect the specimens in sterile vials. Label each specimen container with the patient's ID number and the date collected. If shipping domestically, use cold packs to keep the sample at 4°C. If shipping internationally, pack in dry ice.
- Nasopharyngeal or oropharyngeal swabs
Use only sterile dacron or rayon swabs with plastic shafts. Do not use calcium alginate swabs or swabs with wooden sticks, as they may contain substances that inactivate some viruses and inhibit PCR testing.
- Nasopharyngeal swabs -- Insert a swab into the nostril parallel to the palate. Leave the swab in place for a few seconds to absorb secretions. Swab both nostrils.
- Oropharyngeal swabs -- Swab the posterior pharynx and tonsillar areas, avoiding the tongue.
Place the swabs immediately into sterile vials containing 2 ml of viral transport media. Break the applicator sticks off near the tip to permit tightening of the cap. Label each specimen container with the patient's ID number and the date the sample was collected. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, pack in dry ice.
B. Collecting specimens from the lower respiratory tract
-
Broncheoalveolar lavage, tracheal aspirate, pleural fluid tap
Centrifuge half of the specimen, and fix the cell pellet in formalin. Place the remaining unspun fluid in sterile vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm ® . Label each specimen container with the patient's ID number and the date the sample was collected. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, ship fixed cells at room temperature and unfixed cells frozen.
-
Sputum
Educate the patient about the difference between sputum and oral secretions. Have the patient rinse the mouth with water and then expectorate deep cough sputum directly into a sterile screw-cap sputum collection cup or sterile dry container. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, pack in dry ice.
II. Collecting Blood Components
Serum and blood (plasma) should be collected early in the illness for RT-PCR testing. The reliability of RT-PCR testing performed on blood specimens decreases as the illness progresses.
Both acute and convalescent serum specimens should be collected for antibody testing. To confirm or rule out SARS-CoV infection, it is important to collect convalescent serum specimens >28 days after the onset of illness.
A. Collecting serum for antibody or RT-PCR testing
Collect 5 ml-10 ml of whole blood in a serum separator tube. Allow the blood to clot, centrifuge briefly, and collect all resulting sera in vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm ® . The minimum amount of serum preferred for each test is 200 microliters, which can easily be obtained from 5 mL of whole blood.
A minimum of 1 cc of whole blood is needed for testing of pediatric patients. If possible, collect 1 cc in an EDTA tube and in a clotting tube. If only 1cc can be obtained, use a clotting tube.
Label each specimen container with the patient's ID number and the date the specimen was collected. If unfrozen and transported domestically, ship with cold packs to keep the sample at 4°C. If frozen or transported internationally, ship on dry ice.
B. Collecting EDTA blood (plasma) for RT-PCR
Collect 5 ml-10 ml of blood in an EDTA (purple-top) tube. Transfer to vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm ® . Label each specimen container with patient's ID number and date of collection. Store and ship blood specimens with cold packs to keep the sample at 4°C.
III. Collecting Stool Specimens for PCR
Begin collecting stool specimens as soon as possible in the course of the illness. Although collecting earlier specimens is ideal, SARS-CoV has been detected in stool as late as one month after the onset of symptoms.
Place each stool specimen - as large a quantity as can be obtained (at least 10 cc) - in a leak-proof, clean, dry container, and refrigerate at 4°C. Patients may drape plastic kitchen wrap across the back half of the toilet, under the toilet seat, to facilitate collection of stool specimens.
IMPORTANT: Refrigerate or freeze tubes after specimens are placed in them. If specimens will be examined within 48 hours after collection, they can be refrigerated. If specimens must be held longer than 48 hours, freeze them as soon as possible after collection. Although storage in an ultra-low freezer (-70°C) is preferable, storage in a home-type freezer (if properly set at -20°C) is acceptable for short periods.
Specimens
from possible and known SARS cases must be packaged, shipped,
and transported according to the current edition of the "International
Air Transport Association (IATA) Dangerous Goods Regulations
at www.iata.org/dangerousgoods/index and
US DOT 49 CFR Parts 171-180 at hazmat.dot.gov/rules.htm).
Step-by-step
instructions on appropriate packaging and labeling are available
at www.cdc.gov/ncidod/sars/pdf/packingspecimens-sars.pdf.
Click to open figure in new window
Specimens for SARS-CoV Testing: Priority Specimens and Timing for Collection
The likelihood of detecting infection is increased if multiple specimens, e.g., stool, serum, and respiratory tract specimens, are collected during the course of illness. |
Specimen, by test type |
<1 week after symptom onset |
1-3 weeks after symptom onset |
>3 weeks after symptom onset |
RT-PCR 1 for viral RNA detection |
|
|
|
Sputum 2 |
 3 3
|
 
|

|
Bronchoalveolar lavage, tracheal aspirate, or pleural fluid tap 4 |

|
 
|

|
Nasopharyngeal wash/aspirate |

|
 
|

|
Nasopharyngeal and oropharyngeal swabs |

|
 
|

|
Serum (serum separator tube) |
 
|

|
not recommended |
Blood (plasma)
(EDTA/purple top tube for plasma) |
 
|

|
not recommended |
Stool (minimum 10 cc specimen) |

|
 
|
 
|
EIA 1 for antibody detection |
|
|
|
Serum 5 (serum separator tube) |
 
|
 
|

|
1 Because
of the investigational nature of both the SARS RT-PCR
(reverse transcription-polymerase chain reaction) and
the SARS EIA (enzyme immunoassay), it is recommended that
the clinician obtain a signed informed consent form from
the patient. The consent form for the RT-PCR test can
be found online. The consent form for the EIA test can also be found online.
2 A sputum specimen is preferred if the patient has a productive cough.
3 The more checks, the better the results from a particular specimen at a specific point in the illness.
4 Consider these specimen types if sputum is not available.
5 Also collect a convalescent specimen >28 days post onset.
|
 |