 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Automated Detection and Reporting of Notifiable Diseases Using Electronic Medical Records Versus Passive Surveillance --- Massachusetts, June 2006--July 2007Electronic medical record (EMR) systems have the potential to improve reporting of notifiable diseases beyond either traditional clinician-initiated or automated laboratory-based reporting systems. Traditional clinician-initiated passive surveillance is burdensome to clinicians and often incomplete and delayed (1,2). Electronic laboratory reporting addresses these limitations (3,4) but often lacks information needed for public health purposes (e.g., patient signs and symptoms, prescribed treatments, and pregnancy status). Laboratory systems also do not integrate multiple laboratory tests to satisfy a case definition. Many EMRs, however, contain this information and store it in a form that is amenable to electronic analysis and reporting. Consequently, EMR-based reporting has the potential to provide active notifiable disease surveillance that is more timely, complete, and clinically detailed. This report summarizes findings from a pilot EMR-based electronic surveillance system in Massachusetts, which documented increases of 39% in reported chlamydia and 53% in reported gonorrhea for the period June 2006--July 2007, compared with the existing passive surveillance system. Eighty-one instances of pregnancy not identified by passive surveillance were reported by the electronic system in patients with chlamydia or gonorrhea. In addition, the electronic system identified 20 cases of pelvic inflammatory disease and four cases of acute hepatitis A, compared with none and one, respectively, reported via the passive system. Improved reporting can help public health departments better allocate limited resources for targeted investigations and interventions. The Massachusetts Department of Public Health, the Department of Ambulatory Care and Prevention at Harvard Medical School and Harvard Pilgrim Health Care, and Atrius Health collaborated under the auspices of the CDC Center of Excellence in Public Health Informatics at Harvard Pilgrim Health Care and Children's Hospital in Boston to create a system called Electronic Support for Public Health (ESP) (5). Atrius Health is a multispecialty group practice that provides primary care and outpatient specialty services to approximately 600,000 children and adults at 35 clinical facilities in eastern Massachusetts. ESP is designed to be compatible with most commercial EMR systems. Data on patient visits are exported from the EMR system to an independent ESP server every 24 hours. Transfer of data is an important aspect of ESP's compatibility with most EMR systems. Export of data also enables analysis without burdening the medical practice EMR system. The ESP server is secured behind the practice's electronic firewall to ensure the security of sensitive clinical information. ESP was activated in January 2007 and populated with data beginning in June 2006. Data transferred from the EMR system to ESP include patient demographics, vital signs, test orders, test results, prescriptions, diagnostic codes, and health-care provider details. These data were regularly analyzed by ESP for evidence of four notifiable diseases: chlamydia, gonorrhea, pelvic inflammatory disease, and acute hepatitis A. Cases were defined on the basis of combinations of test orders, test results, medication prescriptions, and International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) diagnostic codes. ESP case definitions were modeled after CDC surveillance definitions but limited to the coded data captured by EMRs (Table). When one of the four notifiable diseases was detected, ESP generated an electronic report and transmitted it to the state health department via the Internet. The report included the patient's name and contact information, clinician's name and contact information, disease diagnosed, laboratory test results, prescriptions, pregnancy status, and any patient symptoms that were inferred from ICD-9 codes in the electronic record. During the study period, the practice's conventional reporting continued routinely, independent of ESP, under the auspices of an infection-control practitioner in some facilities and through spontaneous clinician initiative in others. The practice's personnel were not informed of cases identified by ESP. For the period June 2006--July 2007, ESP reported 758 cases of chlamydia, 95 cases of gonorrhea, 20 cases of pelvic inflammatory disease, and four cases of acute hepatitis A. The charts of all cases identified by ESP were manually reviewed and matched with conventional, passive surveillance case reports in health department records. Compared with passive, paper-based reporting, ESP increased the number of chlamydia reports by 39% (758 cases versus 545) and gonorrhea by 53% (95 cases versus 62). In addition, ESP identified 20 cases of pelvic inflammatory disease cases, compared with none identified by passive surveillance, and four cases of acute hepatitis A, compared with one. A total of six cases of chlamydia in health department records were not detected by ESP. Chart reviews revealed five of these to be false positives. The one true case missed by ESP had been miscoded in the EMR system that fed data to ESP. All cases of gonorrhea, pelvic inflammatory disease, and acute hepatitis A detected by passive surveillance also were identified by ESP. All ESP case reports included patient treatment information and pregnancy status. In contrast, passive surveillance reports included pregnancy status for 5% of cases and treatment information for 88% of cases. ESP reported 81 cases of pregnancy in females with chlamydia or gonorrhea that were not noted on passive surveillance reports. Spellings of patient names on passive surveillance reports were compared with spellings in ESP data. Passive surveillance reports had a 5% rate of transcription errors, compared with no errors in ESP reports. Reported by: M Klompas, MD, R Lazarus, MBBS, MPH, R Platt, MD, Dept of Ambulatory Care and Prevention, Harvard Medical School and Harvard Pilgrim Health Care; X Hou, MSc, Channing Laboratory, Brigham and Women's Hospital and Harvard Medical School; FX Campion, MD, B Kruskal, MD, PhD, Atrius Health and Harvard Medical School, Boston; G Haney, MPH, W Dumas, J Daniel, MPH, A DeMaria, MD, Massachusetts Dept of Public Health. SJN McNabb, PhD, Div of Integrated Surveillance Systems and Services, National Center for Public Health Informatics, CDC. Editorial Note:This comparison of EMR-based detection and reporting of four notifiable diseases with traditional methods illustrates that automated, active surveillance using EMR data has the potential to improve public health monitoring by ensuring that cases are reported and by enhancing the timeliness, accuracy, and clinical detail of reports. Improved reporting can help public health departments better allocate limited resources for targeted investigations and interventions. For example, ESP has the potential to reliably identify high-priority cases for intervention, such as untreated chlamydia or gonorrhea in women who are pregnant. EMR-based surveillance and reporting also might support additional public health practices, such as populating immunization information systems or enabling statistical analyses for outbreak detection and investigation. Currently, EMR-based surveillance cannot obviate the need for additional data collection by public health department personnel for certain cases. EMRs typically do not contain certain pieces of key epidemiologic data in a coded form that can be identified readily by electronic algorithms. Examples include case contacts, risky behaviors, foreign travel, and relevant occupations (e.g., food handler or day care worker). ESP complements the existing National Electronic Diseases Surveillance System (NEDSS), established by CDC in 2001 to enable local and state health departments to send electronic data to CDC for public health monitoring of notifiable conditions nationwide (6). NEDSS-compatible systems currently operate in 38 states and the District of Columbia (7). ESP can facilitate electronic collection and transmission of case reports from health-care providers to local and state public health departments, which in turn can use NEDSS-compatible systems to deliver summary data to CDC. Different strategies for implementing EMR-based case detection and reporting will need to be developed for different kinds of medical organizations. The stand-alone, server-based implementation of ESP described in this report is designed for large practices or health information exchanges. Incorporating ESP logic into commercial EMR software or developing stand-alone ESP modules that can operate side by side with EMRs on clinicians' office-based computers also should be feasible. Source code for ESP is freely available (at http://esphealth.org) under an open source license compatible with commercial development. Widespread implementation of electronic case detection and reporting is currently limited by the slow pace of adoption of EMRs by clinical practices, variation in coding practices among proprietary EMR systems, and an absence of standards for identifying cases using electronic data alone. As of 2006, only 29% of ambulatory medical practices were using EMR systems (8). In addition, the breadth of information collected by EMR systems varies substantially. EMRs that do not include prescription data, for example, limit the sensitivity and specificity of some electronic disease-detection algorithms (9). Further, different EMR systems use different proprietary coding systems. ESP does include tools to translate proprietary codes into standard nomenclatures; however, use of this translation requires customization for each system. Finally, existing case definitions for notifiable diseases (10) incorporate clinical descriptors such as "acute onset of symptoms" or "undue fatigue" or "jaundice" that are inconsistently noted on EMRs. Development of ESP continues. The system has added reporting of acute hepatitis B, acute hepatitis C, and active tuberculosis. In addition, ESP's portability is being assessed by installing it in a regional health information exchange populated by a different EMR system. Meanwhile, development of electronic case definitions and widespread adoption of standard laboratory test nomenclatures, consensus lists of treatments, and standard reporting elements will facilitate more meaningful, comparable, and widespread electronic reporting of notifiable diseases. Acknowledgment This report is based, in part, on the contributions of J Dunn, MPH, Dept of Ambulatory Care and Prevention, Harvard Medical School and Harvard Pilgrim Health Care, Boston, Massachusetts. References
Table 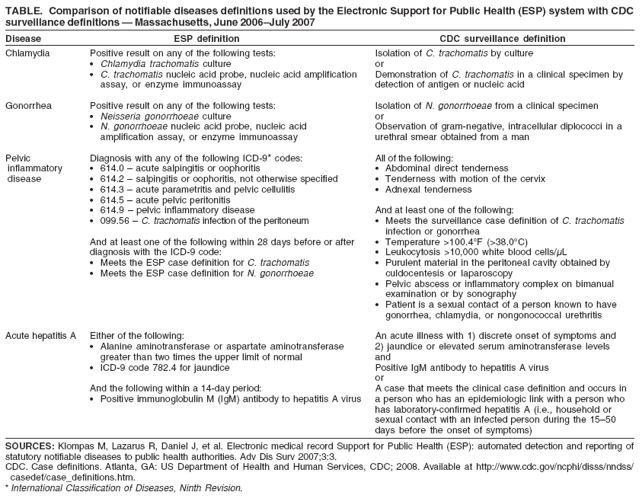 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 4/9/2008 |
|||||||||
|