Vaccines and Preventable Diseases:
Varicella Vaccine - Q&As about Pregnancy
Clinical Questions and Answers
![]()
How long after receiving varicella vaccine should a woman wait to become pregnant?
The ACIP and the AAP recommend that a woman not become pregnant for at least one month following each dose of the vaccine. This differs from the package insert, which recommends a three-month delay.
What are the risks of varicella in pregnant women?
Susceptible pregnant women are at risk for associated complications when they contract varicella. Varicella causes severe maternal morbidity, and 10%-20% of infected women develop varicella pneumonia, with mortality reported as high as 40%.
Newborns whose mothers develop varicella rash from 5 days before to 2 days after delivery are at risk for neonatal varicella, associated with mortality as high as 30%. Furthermore, intrauterine infection may result in stillbirth, herpes zoster during infancy or early childhood, or congenital varicella syndrome.
See also What is recommended for prophylaxis following varicella exposure for pregnant women without evidence of immunity?
What is congenital varicella syndrome?
Infants born to mothers who become infected with varicella during the first half of pregnancy are at risk for a very serious condition known as congenital varicella syndrome, characterized by low birthweight, cutaneous scarring, limb hypoplasia, microcephaly, cortical atrophy, chorioretinitis, cataracts, and other anomalies.
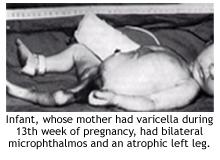
In a prospective study conducted in Europe from 1980-1993 involving nearly 1400 mothers who had varicella during pregnancy, the highest risk (2%) for congenital varicella syndrome was observed when maternal infection occurred during 13-20 weeks’ gestation.
If a woman receives varicella vaccine and subsequently finds out that she is pregnant, what should she be told about the risk to the fetus?
If a pregnant woman is vaccinated or becomes pregnant within 1 month of vaccination, she should be counseled about potential effects on the fetus. Wild-type varicella poses a small risk to the fetus, and because the virulence of the attenuated virus used in the vaccine is less than that of the wild-type virus, the risk to the fetus should be lower or absent. In order to clarify this risk, Merck and Co., Inc., in collaboration with CDC, established the VARIVAX Pregnancy Registry to monitor the maternal-fetal outcomes of pregnant women who were inadvertently administered varicella vaccine 3 months before or any time during pregnancy. During the first 10 years of the pregnancy registry, no cases of congenital varicella syndrome or birth defects compatible with congenital varicella syndrome have been documented.
We encourage enrollment of women who were given varicella vaccine inadvertently three months prior or during pregnancy. Women may be enrolled in the registry by calling 1-800-986-8999.
For more information on the VARIVAX Pregnancy Registry, visit the following site: www.cdc.gov/epo/mmwr/preview/mmwrhtml/00040693.htm
Should children and susceptible persons living in a household with a susceptible pregnant woman be vaccinated?
Yes, all healthy children and adults without evidence of immunity who are living in a household with a susceptible pregnant woman should be vaccinated. Pregnant women without evidence of immunity are at risk of getting chickenpox during pregnancy, and vaccination of household contacts reduces this risk. Although there is a minimal risk that a vaccinated person could get a vaccine rash and spread the vaccine strain virus to the pregnant woman, the benefits of vaccinating susceptible household contacts outweigh the extremely low potential risk for transmission of vaccine virus.
What is recommended for prophylaxis following varicella exposure for pregnant women without evidence of immunity?
Because pregnant women might be at higher risk for severe varicella and complications, use of varicella-zoster immune globulin (VZIG) should be strongly considered for pregnant women without evidence of immunity who have been exposed. However, administration of VZIG to pregnant women has not been found to prevent viremia, and therefore does not prevent fetal infection, congenital varicella syndrome, or neonatal varicella. The primary indication for VZIG in pregnant women is to prevent complications in the mother rather than to protect the fetus.
To reduce the risk of severe disease in infants, ACIP recommends that neonates born to mothers who have signs and symptoms of varicella from 5 days before to 2 days after delivery should receive VZIG, regardless of whether the mother received VZIG.
For premature neonates exposed to varicella postnatally, the following is recommended:
- Those born at or after 28 weeks of gestation whose mothers do not have evidence of immunity should receive VZIG because the infant’s immune system may not be fully functional.
- Those who are less than 28 weeks gestation or who weigh 1,000g or less at birth should receive VZIG regardless of maternal history because such infants may have not acquired maternal antibodies.
How should a pregnant woman with varicella be advised and treated?
Pregnant women with varicella should be counseled on the risks of severe disease in themselves and their infants and the recommended treatment options to reduce these risks. Acyclovir is a synthetic nucleoside analog that inhibits replication of human herpes viruses, including VZV. Acylovir is classified as Category B in the FDA use-in-pregnancy rating. Although studies involving animals have not indicated teratogenic effects, adequate, well-controlled studies of acyclovir among pregnant women have not been conducted. However, a prospective registry of acyclovir use during pregnancy that collected data on outcomes of live births of 522 women exposed to systemic acyclovir during the first trimester of pregnancy indicated that the rate and types of birth defects approximated those in the general population. AAP has not recommended oral acyclovir for pregnant women; however, in instances of serious, viral-mediated complications (e.g., pneumonia), AAP has recommended that intravenous acyclovir should be considered.
In addition, the onset of varicella in pregnant women from 5 days before to 2 days after delivery results in severe varicella infection in an estimated 17%-30% of their newborns. These infants are exposed to VZV without sufficient maternal antibody to lessen the severity of disease, and should be given VZIG as described above.
What should healthcare providers do to ensure pregnant women are protected from varicella?
ACIP recommends prenatal assessment of women for evidence of varicella immunity.
For pregnant women, evidence of immunity includes any of the following:
- Documentation of two doses of varicella vaccine;
- Blood tests showing immunity to varicella or laboratory confirmation of prior disease; or
- Receipt from a healthcare provider of a) a diagnosis of chickenpox or herpes zoster (shingles) or b) verification of a history of chickenpox or herpes zoster (shingles).
Because of the potential severe consequences of varicella infection during pregnancy, birth before 1980 is not considered evidence of immunity for pregnant women.
Healthcare settings serving pregnant women should use standing orders to ensure the administration of varicella vaccine to women without evidence of immunity upon completion or termination of their pregnancies. As soon as the pregnant woman delivers her baby, she should be vaccinated against varicella. The first dose of vaccine can be given before she is discharged from the hospital, and the second dose at the 6-8-week post-partum visit.
Can women who received postpartum varicella vaccination breastfeed their infants?
Yes, neither inactivated nor most live vaccines (including varicella vaccine) administered to a lactating woman affect the safety of breastfeeding for mothers or infants. Breastfeeding does not adversely affect immunization and is not a contraindication for vaccines. NOTE: Vaccinia (smallpox) vaccine is the exception to this rule; vaccinia vaccine is contraindicated for breastfeeding mothers.
For varicella, a study involving 12 women who received single-antigen varicella vaccine while breastfeeding indicated no evidence of VZV DNA either in several hundred breast milk samples collected or in the infants tested after both vaccine doses. Another study did not detect varicella gene sequences in the post-vaccination breast milk samples.
Return to main Varicella Vaccination page
Content last reviewed on June 12, 2007
Content Source: National Center for Immunization and Respiratory Diseases
